α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2
- PMID: 28598982
- PMCID: PMC5466328
- DOI: 10.1371/journal.pone.0179204
α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2
Abstract
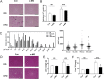
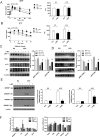
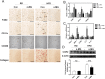
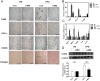
Obesity induces various metabolic diseases such as dyslipidemia, nonalcoholic fatty liver disease (NAFLD), and type 2 diabetes. Fat expansion in adipose tissue induces adipose tissue dysfunction and inflammation, insulin resistance, and other metabolic syndromes. α-Mangostin (α-MG) has been previously studied for its anti-cancer, anti-inflammatory, and antioxidant activities. In this study, we investigated the effects of α-MG on adipose tissue inflammation and hepatic steatosis. We categorized study animals into four groups: regular diet control mice, RD mice treated with α-MG, high fat diet-induced obese mice, and HFD mice treated with α-MG. α-MG treatment significantly reduced not only the body, liver, and fat weights, but also plasma glucose, insulin, and triglyceride levels in HFD mice. Additionally, adiponectin levels of α-MG-treated mice were significantly higher than those of control HFD mice. Immunohistochemistry of liver and adipose tissue showed that CD11c expression was reduced in α-MG fed obese mice. α-MG treatment of HFD mice down-regulated the adipose-associated inflammatory cytokines and CCR2 in both liver and adipose tissue. Moreover, glucose tolerance and insulin sensitivity were significantly improved in α-MG fed obese mice. α-Mangostin ameliorates adipose inflammation and hepatic steatosis in HFD-induced obese mice.
Conflict of interest statement
Figures



References
-
- Yang L, Colditz GA (2015) Prevalence of Overweight and Obesity in the United States, 2007–2012. JAMA Intern Med 175: 1412–1413. doi: 10.1001/jamainternmed.2015.2405 - DOI - PMC - PubMed
-
- Grundy SM (2004) Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab 89: 2595–2600. doi: 10.1210/jc.2004-0372 - DOI - PubMed
-
- Pi-Sunyer FX (1991) Health implications of obesity. Am J Clin Nutr 53: 1595S–1603S. - PubMed
-
- Quan X, Wang Y, Ma X, Liang Y, Tian W, Ma Q, et al. (2012) alpha-Mangostin induces apoptosis and suppresses differentiation of 3T3-L1 cells via inhibiting fatty acid synthase. PLoS One 7: e33376 doi: 10.1371/journal.pone.0033376 - DOI - PMC - PubMed
-
- Pradhan A (2007) Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev 65: S152–156. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

