Coexistence of Eph receptor B1 and ephrin B2 in port-wine stain endothelial progenitor cells contributes to clinicopathological vasculature dilatation
- PMID: 28599054
- PMCID: PMC9375175
- DOI: 10.1111/bjd.15716
Coexistence of Eph receptor B1 and ephrin B2 in port-wine stain endothelial progenitor cells contributes to clinicopathological vasculature dilatation
Abstract
Background: Port-wine stain (PWS) is a vascular malformation characterized by progressive dilatation of postcapillary venules, but the molecular pathogenesis remains obscure.
Objectives: To illustrate that PWS endothelial cells (ECs) present a unique molecular phenotype that leads to pathoanatomical PWS vasculatures.
Methods: Immunohistochemistry and transmission electron microscopy were used to characterize the ultrastructure and molecular phenotypes of PWS blood vessels. Primary culture of human dermal microvascular endothelial cells and in vitro tube formation assay were used for confirmative functional studies.
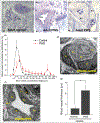
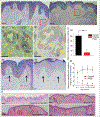
Results: Multiple clinicopathological features of PWS blood vessels during the development and progression of the disease were shown. There were no normal arterioles and venules observed phenotypically and morphologically in PWS skin; arterioles and venules both showed differentiation impairments, resulting in a reduction of arteriole-like vasculatures and defects in capillary loop formation in PWS lesions. PWS ECs showed stemness properties with expression of endothelial progenitor cell markers CD133 and CD166 in non-nodular lesions. They also expressed dual venous/arterial identities, Eph receptor B1 (EphB1) and ephrin B2 (EfnB2). Co-expression of EphB1 and EfnB2 in normal human dermal microvascular ECs led to the formation of PWS-like vasculatures in vitro, for example larger-diameter and thick-walled capillaries.
Conclusions: PWS ECs are differentiation-impaired, late-stage endothelial progenitor cells with a specific phenotype of CD133+ /CD166+ /EphB1+ /EfnB2+ , which form immature venule-like pathoanatomical vasculatures. The disruption of normal EC-EC interactions by coexistence of EphB1 and EfnB2 contributes to progressive dilatation of PWS vasculatures.
© 2017 British Association of Dermatologists.
Conflict of interest statement
Conflicts of interest
None declared.
Figures


Comment in
-
B1 and B2: a role for ephrin signalling in port-wine stain.Br J Dermatol. 2017 Dec;177(6):1478-1479. doi: 10.1111/bjd.16035. Br J Dermatol. 2017. PMID: 29313918 No abstract available.
References
-
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics 1976; 58:218–22. - PubMed
-
- Pratt AG. Birthmarks in infants. Arch Dermatol Syphilol 1953; 67:302–5. - PubMed
-
- Geronemus RG, Ashinoff R. The medical necessity of evaluation and treatment of port-wine stains. J Dermatol Surg Oncol 1991; 17:76–9. - PubMed
-
- Lever WF, Schaumburg-Lever G. Histopathology of the Skin, 7th edn. Philadelphia, PA: JB Lippincott, 1990.
-
- Kalick SM. Toward an interdisciplinary psychology of appearances. Psychiatry 1978; 41:243–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

