Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: a randomized placebo-controlled trial
- PMID: 28599630
- PMCID: PMC5466796
- DOI: 10.1186/s12884-017-1365-x
Omega-3 supplementation from pregnancy to postpartum to prevent depressive symptoms: a randomized placebo-controlled trial
Abstract
Background: Low n-3 polyunsaturated fatty acids (PUFAs) has been linked to depression, but the preventive effect of n-3PUFAs supplementation on maternal depression needs further investigation. We aimed to evaluate the efficacy of a daily dose of n-3 PUFAs supplementation (fish oil) on the prevention of postpartum depression (PPD).
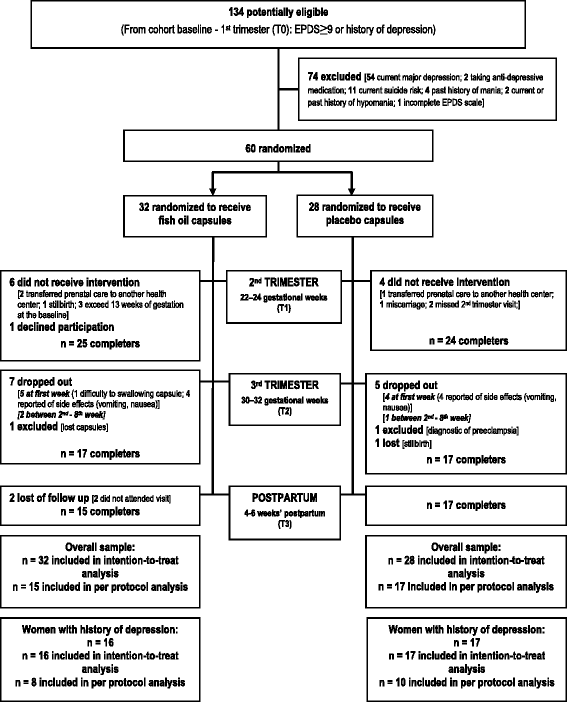
Methods: A randomized, placebo-controlled, double blind trial was designed and nested into a cohort study conducted in Rio de Janeiro, Brazil. Sixty pregnant women identified as being at risk for PPD were invited and randomly assigned to receive fish oil capsules [1.8 g (1.08 g of Eicosapentaenoic (EPA) and 0.72 g of Docosapentaenoic (DHA) acids)] or placebo (control). The Edinburgh Postnatal Depression Scale (EPDS) was scored at 5-13 (T0, baseline), 22-24 (T1), 30-32 weeks of gestation (T2) and 4-6 weeks' postpartum (T3). Supplementation started at week 22-24 of gestation (T1) and lasted for 16 weeks. Serum fatty acids were assayed to evaluate compliance. Prevalence of EPDS ≥11 was the primary outcome, and mean and changes in EPDS score, length of gestation, and birth weight the secondary outcomes. Linear mixed-effect (LME) and random-intercept logistic regression models were performed to test the effect of fish oil supplementation on prevalence of EPDS ≥11 and EPDS scores variation.
Results: In intention-to-treat (ITT) analysis, at 30-32 weeks' gestation women in the fish oil presented higher serum concentration of EPA, DHA and lower n-6/n-3 ratio comparing to the control group. There were no differences between intervention and control groups in the prevalence of EPDS ≥11, EPDS scores over time, or in changes in EPDS scores from pregnancy to postpartum in either the ITT or per-protocol analyses. Women in the fish oil group with previous history of depression presented a higher reduction on the EPDS score from the second to the third trimester in the fish oil comparing to the control group in the ITT analyses [-1.0 (-3.0-0.0) vs. -0.0 (-1.0-3.0), P = 0.038). These results were confirmed on the LME model (β = -3.441; 95%CI: -6.532- -0.350, P = 0.029).
Conclusion: Daily supplementation of 1.8 g of n-3 PUFAs during 16 weeks did not prevent maternal depressive symptoms in a sample of Brazilian women.
Trial registration: ClinicalTrials.gov Identifier: NCT01660165 . Retrospectively registered on 24 May 2012.
Keywords: Depression; pregnancy; Fatty acids; Omega-3; Randomized controlled trial.
Figures

References
-
- Vaz Jdos S, Kac G, Emmett P, Davis JM, Golding J, Hibbeln JR. Dietary patterns, n-3 fatty acids intake from seafood and high levels of anxiety symptoms during pregnancy: findings from the Avon longitudinal study of parents and children. PLoS One. 2013;8(7):e67671. doi: 10.1371/journal.pone.0067671. - DOI - PMC - PubMed
-
- Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr. 2011;7(Suppl 2):17–26. doi: 10.1111/j.1740-8709.2011.00299.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

