Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology
- PMID: 28599681
- PMCID: PMC5466770
- DOI: 10.1186/s40478-017-0445-5
Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology
Erratum in
-
Correction to: Brain-derived exosomes from dementia with Lewy bodies propagate α-synuclein pathology.Acta Neuropathol Commun. 2020 Aug 4;8(1):123. doi: 10.1186/s40478-020-01006-4. Acta Neuropathol Commun. 2020. PMID: 32753049 Free PMC article.
Abstract
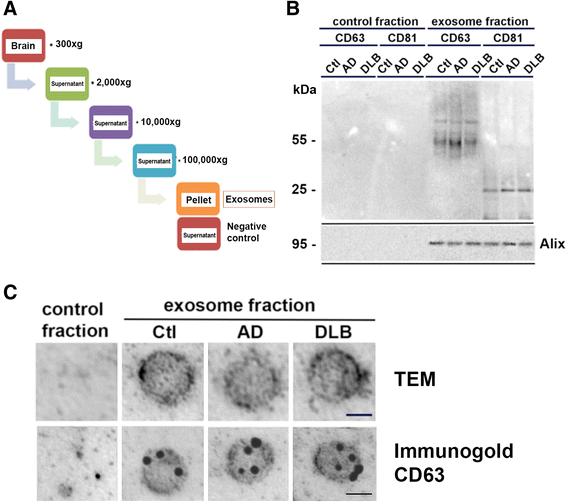
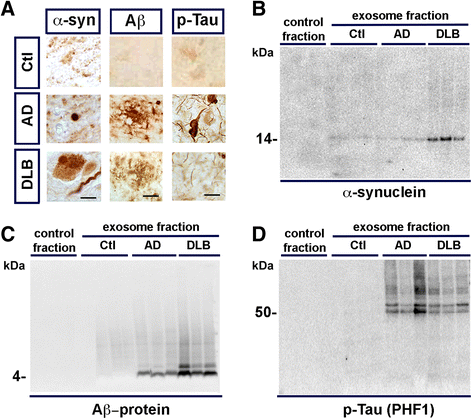
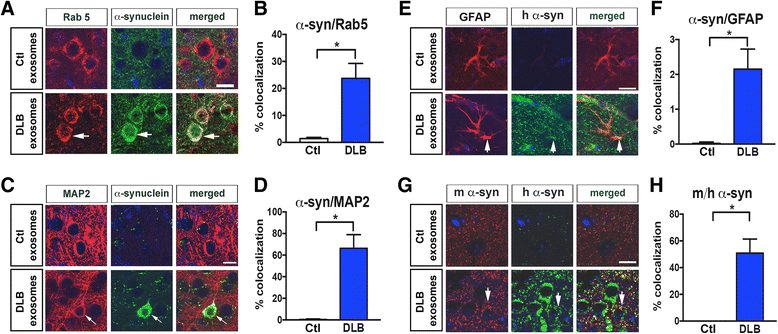
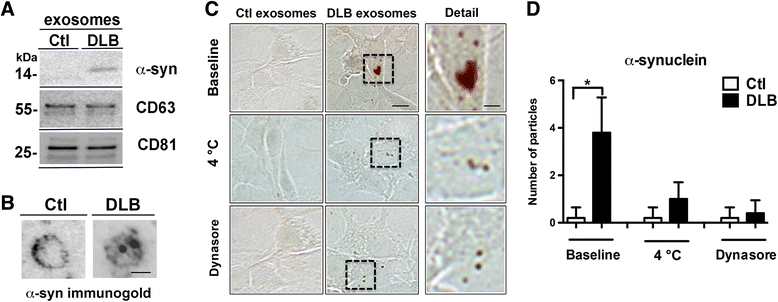
Proteins implicated in neurodegenerative conditions such as Alzheimer's disease (AD) and Dementia with Lewy Bodies (DLB) have been identified in bodily fluids encased in extracellular vesicles called exosomes. Whether exosomes found in DLB patients can transmit pathology is not clear. In this study, exosomes were successfully harvested through ultracentrifugation from brain tissue from DLB and AD patients as well as non-diseased brain tissue. Exosomes extracted from brains diagnosed with either AD or DLB contained aggregate-prone proteins. Furthermore, injection of brain-derived exosomes from DLB patients into the brains of wild type mice induced α-synuclein (α-syn) aggregation. As assessed through immunofluorescent double labeling, α-syn aggregation was observed in MAP2+, Rab5+ neurons. Using a neuronal cell line, we also identified intracellular α-syn aggregation mediated by exosomes is dependent on recipient cell endocytosis. Together, these data suggest that exosomes from DLB patients are sufficient for seeding and propagating α-syn aggregation in vivo.
Keywords: Alpha synuclein; Alzheimer’s disease; Amyloid beta; Dementia; Exosomes; Lewy body; Tau.
Figures

References
-
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ (1994) Induction of β (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Mol Neurobiol 8:25–39. doi:10.1007/BF02778005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

