Planar cell polarity-dependent and independent functions in the emergence of tissue-scale hair follicle patterns
- PMID: 28599846
- PMCID: PMC5549468
- DOI: 10.1016/j.ydbio.2017.06.003
Planar cell polarity-dependent and independent functions in the emergence of tissue-scale hair follicle patterns
Abstract
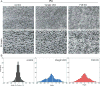
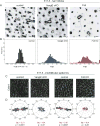
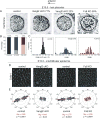
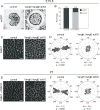
Hair follicles of the mammalian epidermis display local order and global alignment, a complex pattern instructed by the core planar cell polarity (PCP) pathway. Here we address the contributions of core PCP genes, Van Gogh-like and Frizzled, to the establishment, local refinement, and global order of embryonic and postnatal hair follicles. We find that, similar to Fz6 mutants, the disordered hair patterns of Vangl2 mutants are refined over time and eventually corrected. In both mutants, we find that tissue-level reorientation occurs through locally coordinated follicle rotation at stereotyped locations. Strikingly, Vangl2 and Fz6 mutant follicles collectively rotate with opposing directionalities, suggesting that redundant core PCP signals contribute to their directed realignment. Consistently, global follicle alignment is not restored upon conditional ablation of both Vangl1 and Vangl2 genes. Instead, spatially distinct patterns of whorls and crosses emerge and persist even after a complete cycle of hair follicle regeneration. Thus, local refinement of hair follicles into higher order patterns can occur independently of the core PCP system, however, their global alignment with the body axes requires PCP function throughout morphogenesis, growth and regeneration.
Keywords: Celsr1; Epidermis; Fz6; Hair follicles; Planar cell polarity; Vangl2.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures






References
-
- Adler PN, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. - PubMed
-
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. - PubMed
-
- Alonso L, Fuchs E. The hair cycle. J. Cell Sci. 2006;119:391–393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

