Impact of aspirin on fetal growth in diabetic pregnancies according to White classification
- PMID: 28599894
- PMCID: PMC5737770
- DOI: 10.1016/j.ajog.2017.05.062
Impact of aspirin on fetal growth in diabetic pregnancies according to White classification
Abstract
Background: Current US Preventive Services Task Force and other guidelines recommend low-dose aspirin for all pregnant women with pregestational diabetes mellitus to prevent preeclampsia and small-for-gestational-age birth. The Maternal-Fetal Medicine Units High-Risk Aspirin trial did not show a reduction in either preeclampsia or small-for-gestational-age birth in diabetic women.
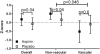
Objective: Our objective was to reassess the impact of aspirin on fetal growth in diabetic pregnancies overall and according to White classification. We hypothesized that aspirin improves fetal growth in pregnancies with vascular complications of diabetes at highest risk for poor fetal growth.
Study design: We conducted secondary analysis of the cohort of diabetic women enrolled in the Maternal-Fetal Medicine Units High-Risk Aspirin trial. The impact of aspirin prophylaxis on birthweight was assessed in the overall cohort and in 2 groups categorized according to White classification as nonvascular (White class B, C, D) or vascular (White class R, F, RF). Birthweight was converted to Z-score normalized for gestational age at delivery and neonatal sex. Difference in birthweight Z-score between aspirin and placebo was tested with a 2-sample t test. The effect of vascular group, aspirin vs placebo randomization, and the interaction of the 2 on normalized birthweight percentile was estimated with linear regression with a multivariable model including covariates body mass index, tobacco use, race, and parity. The percentage of small and large-for-gestational-age newborns born to aspirin- vs placebo-treated women was compared between groups using Pearson exact χ2 analysis, and an adjusted model was estimated by logistic regression.
Results: All 444 women with pregestational diabetes and complete outcome data were included (53 vascular, 391 nonvascular). Aspirin was significantly associated with a higher birthweight Z-score (0.283; 95% confidence interval, 0.023-0.544) in the overall cohort (P = .03). In the adjusted model, the association of aspirin with higher birthweight Z-score was confined to neonates of women with nonvascular diabetes (0.341; 95% confidence interval, 0.677-0.006; P = .044). An opposite but nonsignificant effect was observed among neonates from women with vascular diabetes (-0.416; 95% confidence interval, -1.335 to 0.503; P = .6). This difference in the relationship of aspirin and birthweight Z-score by vascular group was significant at P = .046. Aspirin-randomized women with nonvascular diabetes had more large-for-gestational-age births than those treated with placebo (40.2 vs 26.6%; P = .005). Small-for-gestational-age births occurred at the same frequency with aspirin vs placebo randomization in the overall cohort (8% in each group) and in each vascular group.
Conclusion: Inconsistent with our hypothesis, aspirin did not reduce small-for-gestational-age births in the overall cohort or either group. The increased incidence of large-for-gestational-age infants in aspirin-treated diabetic gestations is of potential concern given the known increased maternal and neonatal morbidity associated with macrosomia.
Keywords: aspirin; diabetes; fetal growth; large for gestational age; macrosomia.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflict of interest.
Figures



References
-
- LeFevre ML, US Preventive Services Task Force Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819–26. - PubMed
-
- Organization WH. WHO recommendations for prevention and treatment of preeclampsia and eclampsia. Geneva: World Health Organization; 2014.
-
- Redman CW. Hypertension in pregnancy: the NICE guidelines. Heart. 2011;97:1967–9. - PubMed
-
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. [Accessed July 12, 2017];2016 Available at: https://www.acog.org/About-ACOG/News-Room/Practice-Advisories/Practice-A....
-
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

