The structural basis of alpha-tropomyosin linked (Asp230Asn) familial dilated cardiomyopathy
- PMID: 28600229
- PMCID: PMC5899615
- DOI: 10.1016/j.yjmcc.2017.06.001
The structural basis of alpha-tropomyosin linked (Asp230Asn) familial dilated cardiomyopathy
Abstract
Recently, linkage analysis of two large unrelated multigenerational families identified a novel dilated cardiomyopathy (DCM)-linked mutation in the gene coding for alpha-tropomyosin (TPM1) resulting in the substitution of an aspartic acid for an asparagine (at residue 230). To determine how a single amino acid mutation in α-tropomyosin (Tm) can lead to a highly penetrant DCM we generated a novel transgenic mouse model carrying the D230N mutation. The resultant mouse model strongly phenocopied the early onset of cardiomyopathic remodeling observed in patients as significant systolic dysfunction was observed by 2months of age. To determine the precise cellular mechanism(s) leading to the observed cardiac pathology we examined the effect of the mutation on Ca2+ handling in isolated myocytes and myofilament activation in vitro. D230N-Tm filaments exhibited a reduced Ca2+ sensitivity of sliding velocity. This decrease in sensitivity was coupled to increase in the peak amplitude of Ca2+ transients. While significant, and consistent with other DCMs, these measurements are comprised of complex inputs and did not provide sufficient experimental resolution. We then assessed the primary structural effects of D230N-Tm. Measurements of the thermal unfolding of D230N-Tm vs WT-Tm revealed an increase in stability primarily affecting the C-terminus of the Tm coiled-coil. We conclude that the D230N-Tm mutation induces a decrease in flexibility of the C-terminus via propagation through the helical structure of the protein, thus decreasing the flexibility of the Tm overlap and impairing its ability to regulate contraction. Understanding this unique structural mechanism could provide novel targets for eventual therapeutic interventions in patients with Tm-linked cardiomyopathies.
Keywords: Cardiomyopathy; Dilated; Mice; Mutation; Transgenic; Tropomyosin.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures




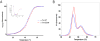
Similar articles
-
Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity.Circ Res. 2007 Jul 20;101(2):205-14. doi: 10.1161/CIRCRESAHA.107.148379. Epub 2007 Jun 7. Circ Res. 2007. PMID: 17556658
-
Clinically Divergent Mutation Effects on the Structure and Function of the Human Cardiac Tropomyosin Overlap.Biochemistry. 2017 Jul 5;56(26):3403-3413. doi: 10.1021/acs.biochem.7b00266. Epub 2017 Jun 21. Biochemistry. 2017. PMID: 28603979 Free PMC article.
-
Familial dilated cardiomyopathy mutations uncouple troponin I phosphorylation from changes in myofibrillar Ca²⁺ sensitivity.Cardiovasc Res. 2013 Jul 1;99(1):65-73. doi: 10.1093/cvr/cvt071. Epub 2013 Mar 27. Cardiovasc Res. 2013. PMID: 23539503
-
Alpha-tropomyosin mutations in inherited cardiomyopathies.J Muscle Res Cell Motil. 2013 Aug;34(3-4):285-94. doi: 10.1007/s10974-013-9358-5. Epub 2013 Sep 5. J Muscle Res Cell Motil. 2013. PMID: 24005378 Review.
-
Biochemical characterisation of Troponin C mutations causing hypertrophic and dilated cardiomyopathies.J Muscle Res Cell Motil. 2014 Apr;35(2):161-78. doi: 10.1007/s10974-014-9382-0. Epub 2014 Apr 18. J Muscle Res Cell Motil. 2014. PMID: 24744096 Review.
Cited by
-
Sex-Specific Effects of MTSS1 Downregulation on Dilated Cardiomyopathy.Circulation. 2024 Nov 12;150(20):1648-1651. doi: 10.1161/CIRCULATIONAHA.124.070418. Epub 2024 Nov 11. Circulation. 2024. PMID: 39527655 No abstract available.
-
Biophysical Derangements in Genetic Cardiomyopathies.Heart Fail Clin. 2018 Apr;14(2):147-159. doi: 10.1016/j.hfc.2017.12.002. Heart Fail Clin. 2018. PMID: 29525644 Free PMC article. Review.
-
Sex-Specific Effect of MTSS1 Downregulation on Dilated Cardiomyopathy.medRxiv [Preprint]. 2024 Mar 1:2024.02.28.24303451. doi: 10.1101/2024.02.28.24303451. medRxiv. 2024. Update in: Circulation. 2024 Nov 12;150(20):1648-1651. doi: 10.1161/CIRCULATIONAHA.124.070418. PMID: 38464240 Free PMC article. Updated. Preprint.
-
Myofilament-associated proteins with intrinsic disorder (MAPIDs) and their resolution by computational modeling.Q Rev Biophys. 2023 Jan 11;56:e2. doi: 10.1017/S003358352300001X. Q Rev Biophys. 2023. PMID: 36628457 Free PMC article. Review.
-
Association of single nucleotide polymorphisms in the 3'UTR region of TPM1 gene with dilated cardiomyopathy: A case-control study.Medicine (Baltimore). 2019 Nov;98(44):e17710. doi: 10.1097/MD.0000000000017710. Medicine (Baltimore). 2019. PMID: 31689804 Free PMC article.
References
-
- Geisterfer-Lowrance A, Kass S, Tanigawa G, Vosberg H, McKenna W, Seidman C, et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. - PubMed
-
- Robinson P, Griffiths PJ, Watkins H, Redwood CS. Dilated and Hypertrophic Cardiomyopathy Mutations in Troponin and α-Tropomyosin Have Opposing Effects on the Calcium Affinity of Cardiac Thin Filaments. Circulation Research. 2007;101:1266–73. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

