Reconstitution of the oocyte nucleolus in mice through a single nucleolar protein, NPM2
- PMID: 28600324
- PMCID: PMC6139372
- DOI: 10.1242/jcs.195875
Reconstitution of the oocyte nucleolus in mice through a single nucleolar protein, NPM2
Abstract
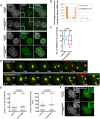
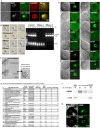
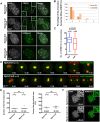
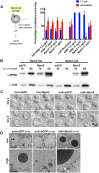
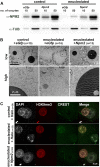
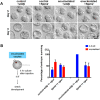
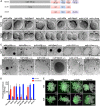
The mammalian oocyte nucleolus, the most prominent subcellular organelle in the oocyte, is vital in early development, yet its key functions and constituents remain unclear. We show here that the parthenotes/zygotes derived from enucleolated oocytes exhibited abnormal heterochromatin formation around parental pericentromeric DNAs, which led to a significant mitotic delay and frequent chromosome mis-segregation upon the first mitotic division. A proteomic analysis identified nucleoplasmin 2 (NPM2) as a dominant component of the oocyte nucleolus. Consistently, Npm2-deficient oocytes, which lack a normal nucleolar structure, showed chromosome segregation defects similar to those in enucleolated oocytes, suggesting that nucleolar loss, rather than micromanipulation-related damage to the genome, leads to a disorganization of higher-order chromatin structure in pronuclei and frequent chromosome mis-segregation during the first mitosis. Strikingly, expression of NPM2 alone sufficed to reconstitute the nucleolar structure in enucleolated embryos, and rescued their first mitotic division and full-term development. The nucleolus rescue through NPM2 required the pentamer formation and both the N- and C-terminal domains. Our findings demonstrate that the NPM2-based oocyte nucleolus is an essential platform for parental chromatin organization in early embryonic development.
Keywords: Heterochromatin; Nucleolus; Nucleoplasmin 2; Oocyte.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
References
-
- Arney K. L., Bao S., Bannister A. J., Kouzarides T. and Surani M. A. (2002). Histone methylation defines epigenetic asymmetry in the mouse zygote. Int. J. Dev. Biol. 46, 317-320. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

