Human RELA haploinsufficiency results in autosomal-dominant chronic mucocutaneous ulceration
- PMID: 28600438
- PMCID: PMC5502421
- DOI: 10.1084/jem.20160724
Human RELA haploinsufficiency results in autosomal-dominant chronic mucocutaneous ulceration
Abstract
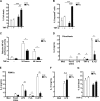
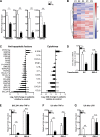
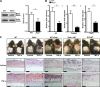
The treatment of chronic mucocutaneous ulceration is challenging, and only some patients respond selectively to inhibitors of tumor necrosis factor-α (TNF). TNF activates opposing pathways leading to caspase-8-mediated apoptosis as well as nuclear factor κB (NF-κB)-dependent cell survival. We investigated the etiology of autosomal-dominant, mucocutaneous ulceration in a family whose proband was dependent on anti-TNF therapy for sustained remission. A heterozygous mutation in RELA, encoding the NF-κB subunit RelA, segregated with the disease phenotype and resulted in RelA haploinsufficiency. The patients' fibroblasts exhibited increased apoptosis in response to TNF, impaired NF-κB activation, and defective expression of NF-κB-dependent antiapoptotic genes. Rela+/- mice have similarly impaired NF-κB activation, develop cutaneous ulceration from TNF exposure, and exhibit severe dextran sodium sulfate-induced colitis, ameliorated by TNF inhibition. These findings demonstrate an essential contribution of biallelic RELA expression in protecting stromal cells from TNF-mediated cell death, thus delineating the mechanisms driving the effectiveness of TNF inhibition in this disease.
© 2017 Badran et al.
Figures


References
-
- Alcamo E., Mizgerd J.P., Horwitz B.H., Bronson R., Beg A.A., Scott M., Doerschuk C.M., Hynes R.O., and Baltimore D.. 2001. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κB in leukocyte recruitment. J. Immunol. 167:1592–1600. 10.4049/jimmunol.167.3.1592 - DOI - PubMed
-
- Boisson B., Laplantine E., Prando C., Giliani S., Israelsson E., Xu Z., Abhyankar A., Israël L., Trevejo-Nunez G., Bogunovic D., et al. 2012. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 13:1178–1186. 10.1038/ni.2457 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

