REG3β modifies cell tumor function by impairing extracellular vesicle uptake
- PMID: 28600520
- PMCID: PMC5466682
- DOI: 10.1038/s41598-017-03244-4
REG3β modifies cell tumor function by impairing extracellular vesicle uptake
Abstract
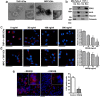
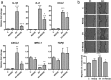
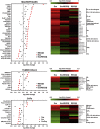
Extracellular vesicles (EVs), including exosomes and microvesicles, are nano-sized membrane vesicles containing proteins and nucleic acids, which act as intercellular messengers. They play an important role in a variety of physiological processes, as well as in pathological situations such as inflammation or cancer. Here, we show that in the case of pancreatic ductal adenocarcinoma (PDAC), the healthy pancreatic tissue surrounding the tumor releases REG3β, a lectin that binds to the glycoproteins present in the surface of EVs, thus interfering with their uptake and internalization by target cells. In vitro, the disruption of the signaling mediated by EVs due to the presence of REG3β, prevents the EV-induced phenotypic switch in macrophages, inhibits the increased cell migration of cancer cells and reverses a number of metabolomic changes promoted by EVs. In vivo, the uptake of REG3β+ EVs by tumor cells is significantly impaired. Furthermore, it results in an increase of circulating REG3β+ EVs in blood of pancreatic cancer patients. Our findings highlight the effect of a lectin released by the healthy pancreatic tissue surrounding the tumor in modulating the EV-mediated interactions between different cell types in PDAC.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

