Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of α V β 3 Integrin Activity
- PMID: 28600529
- PMCID: PMC5466598
- DOI: 10.1038/s41598-017-03224-8
Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of α V β 3 Integrin Activity
Abstract
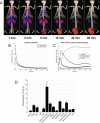
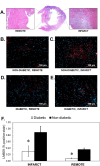
Cyclic peptides containing the Arg-Gly-Asp (RGD) sequence have been shown to specifically bind the angiogenesis biomarker α V β 3 integrin. We report the synthesis, chemical characterization, and biological evaluation of two novel dimeric cyclic RGD-based molecular probes for the targeted imaging of α V β 3 activity (a radiolabeled version, 64Cu-NOTA-PEG4-cRGD2, for PET imaging, and a fluorescent version, FITC-PEG4-cRGD2, for in vitro work). We investigated the performance of this probe at the receptor, cell, organ, and whole-body levels, including its use to detect diabetes associated impairment of ischemia-induced myocardial angiogenesis. Both versions of the probe were found to be stable, demonstrated fast receptor association constants, and showed high specificity for α V β 3 in HUVECs (K d ~ 35 nM). Dynamic PET-CT imaging indicated rapid blood clearance via kidney filtration, and accumulation within α V β 3-positive infarcted myocardium. 64Cu-NOTA-PEG4-cRGD2 demonstrated a favorable biodistribution, slow washout, and excellent performance with respect to the quality of the PET-CT images obtained. Importantly, the ratio of probe uptake in infarcted heart tissue compared to normal tissue was significantly higher in non-diabetic rats than in diabetic ones. Overall, our probes are promising agents for non-invasive quantitative imaging of α V β 3 expression, both in vitro and in vivo.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Evaluation of a dimeric-cRGD peptide for targeted PET-CT imaging of peripheral angiogenesis in diabetic mice.Sci Rep. 2018 Mar 29;8(1):5401. doi: 10.1038/s41598-018-23372-9. Sci Rep. 2018. PMID: 29599497 Free PMC article.
-
(68)Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin alphavbeta3 PET imaging.Eur J Nucl Med Mol Imaging. 2009 Jun;36(6):947-57. doi: 10.1007/s00259-008-1045-1. Epub 2009 Jan 22. Eur J Nucl Med Mol Imaging. 2009. PMID: 19159928
-
Ligands for mapping alphavbeta3-integrin expression in vivo.Acc Chem Res. 2009 Jul 21;42(7):969-80. doi: 10.1021/ar800243b. Acc Chem Res. 2009. PMID: 19489579
-
Radiolabeled RGD peptides as integrin alpha(v)beta3-targeted PET tracers.Curr Med Chem. 2012;19(20):3301-9. doi: 10.2174/092986712801215937. Curr Med Chem. 2012. PMID: 22664242 Review.
-
[Structure-activity relationship studies on cyclic RGD peptides utilizing novel alkene dipeptide isosteres].Yakugaku Zasshi. 2004 May;124(5):269-77. doi: 10.1248/yakushi.124.269. Yakugaku Zasshi. 2004. PMID: 15118239 Review. Japanese.
Cited by
-
[68Ga]-NODAGA-RGD Positron Emission Tomography (PET) for Assessment of Post Myocardial Infarction Angiogenesis as a Predictor for Left Ventricular Remodeling in Mice after Cardiac Stem Cell Therapy.Cells. 2020 May 30;9(6):1358. doi: 10.3390/cells9061358. Cells. 2020. PMID: 32486211 Free PMC article.
-
Multimodal Assessment of Mesenchymal Stem Cell Therapy for Diabetic Vascular Complications.Theranostics. 2017 Sep 5;7(16):3876-3888. doi: 10.7150/thno.19547. eCollection 2017. Theranostics. 2017. PMID: 29109784 Free PMC article.
-
[68Ga]Ga-NODAGA-E[(cRGDyK)]2 angiogenesis PET following myocardial infarction in an experimental rat model predicts cardiac functional parameters and development of heart failure.J Nucl Cardiol. 2023 Oct;30(5):2073-2084. doi: 10.1007/s12350-023-03265-9. Epub 2023 May 1. J Nucl Cardiol. 2023. PMID: 37127725 Free PMC article.
-
Radioiodinated Bicyclic RGD Peptide Derivatives for Enhanced Tumor Accumulation.Pharmaceuticals (Basel). 2025 Apr 8;18(4):549. doi: 10.3390/ph18040549. Pharmaceuticals (Basel). 2025. PMID: 40283983 Free PMC article.
-
Evaluation of a dimeric-cRGD peptide for targeted PET-CT imaging of peripheral angiogenesis in diabetic mice.Sci Rep. 2018 Mar 29;8(1):5401. doi: 10.1038/s41598-018-23372-9. Sci Rep. 2018. PMID: 29599497 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

