Granulin-epithelin precursor interacts with 78-kDa glucose-regulated protein in hepatocellular carcinoma
- PMID: 28601093
- PMCID: PMC5466756
- DOI: 10.1186/s12885-017-3399-x
Granulin-epithelin precursor interacts with 78-kDa glucose-regulated protein in hepatocellular carcinoma
Abstract
Background: Granulin-epithelin precursor (GEP) is a secretory growth factor, which has been demonstrated to control cancer growth, invasion, drug resistance and immune escape. Our previous studies and others also demonstrated its potential in targeted therapy. Comprehensive characterization of GEP partner on cancer cells are warranted. We have previously shown that GEP interacted with heparan sulfate on the surface of liver cancer cells and the interaction is crucial for GEP-mediated signaling transduction. This study aims to characterize GEP protein partner at the cell membrane with the co-immunoprecipitation and mass spectrometry approach.
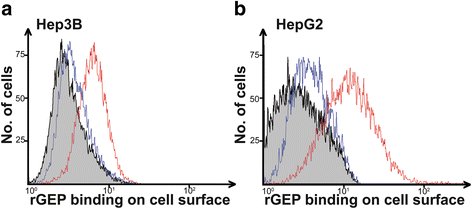
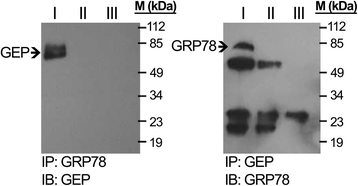
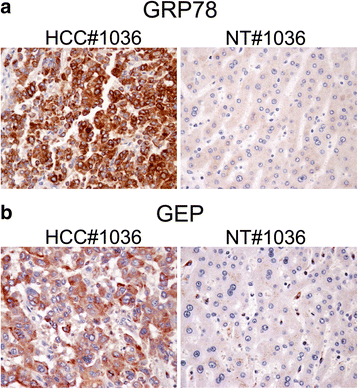
Methods: The membrane fraction from liver cancer model Hep3B was used for capturing binding partner with the specific monoclonal antibody against GEP. The precipitated proteins were analyzed by mass spectrometry. After identifying the GEP binding partner, this specific interaction was validated in additional liver cancer cell line HepG2 by co-immunoprecipitation using GRP78 and GEP antibodies, respectively, as the bait. GRP78 transcript levels in hepatocellular carcinoma (HCC) clinical samples (n = 77 pairs) were examined by real-time quantitative RT-PCR. GEP and GRP78 protein expressions were investigated by immunohistochemistry on paraffin sections.
Results: We identified the GEP-binding protein as 78-kDa glucose-regulated protein (GRP78, also named heat shock 70-kDa protein 5, HSPA5). This interaction was validated in independent HCC cell lines. Increased GRP78 mRNA levels were demonstrated in liver cancer tissues compared with the paralleled liver tissues (t-test, P = 0.002). GRP78 and GEP transcript levels were significantly correlated (Spearman's correlation, P = 0.001), and the proteins were also detectable in the cytoplasm of liver cancer cells by immunohistochemical staining.
Conclusions: GRP78 and GEP are interacting protein partners in liver cancer cells and may play a role in GEP-mediated cancer progression in HCC.
Keywords: 78-kDa glucose-regulated protein; Granulin-epithelin precursor; Hepatocellular carcinoma; Mass spectrometry; Protein interaction.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

