TrmD: A Methyl Transferase for tRNA Methylation With m1G37
- PMID: 28601227
- PMCID: PMC6054489
- DOI: 10.1016/bs.enz.2017.03.003
TrmD: A Methyl Transferase for tRNA Methylation With m1G37
Abstract
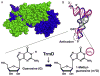
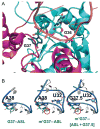
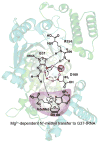
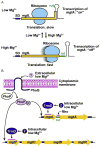
TrmD is an S-adenosyl methionine (AdoMet)-dependent methyl transferase that synthesizes the methylated m1G37 in tRNA. TrmD is specific to and essential for bacterial growth, and it is fundamentally distinct from its eukaryotic and archaeal counterpart Trm5. TrmD is unusual by using a topological protein knot to bind AdoMet. Despite its restricted mobility, the TrmD knot has complex dynamics necessary to transmit the signal of AdoMet binding to promote tRNA binding and methyl transfer. Mutations in the TrmD knot block this intramolecular signaling and decrease the synthesis of m1G37-tRNA, prompting ribosomes to +1-frameshifts and premature termination of protein synthesis. TrmD is unique among AdoMet-dependent methyl transferases in that it requires Mg2+ in the catalytic mechanism. This Mg2+ dependence is important for regulating Mg2+ transport to Salmonella for survival of the pathogen in the host cell. The strict conservation of TrmD among bacterial species suggests that a better characterization of its enzymology and biology will have a broad impact on our understanding of bacterial pathogenesis.
Keywords: Frameshifts; Mg(2+) transport; Protein trefoil knot; S-adenosyl methionine; m(1)G37-tRNA; tRNA(Pro).
© 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Forsyth RA, Haselbeck RJ, Ohlsen KL, Yamamoto RT, Xu H, Trawick JD, Wall D, Wang L, Brown-Driver V, Froelich JM, KGC, King P, et al. A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol Microbiol. 2002;43:1387–1400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
