Impact of the introduction of pneumococcal conjugate vaccination on pneumonia in The Gambia: population-based surveillance and case-control studies
- PMID: 28601421
- PMCID: PMC5589209
- DOI: 10.1016/S1473-3099(17)30321-3
Impact of the introduction of pneumococcal conjugate vaccination on pneumonia in The Gambia: population-based surveillance and case-control studies
Erratum in
-
Corrections.Lancet Infect Dis. 2018 Jul;18(7):715. doi: 10.1016/S1473-3099(18)30363-3. Lancet Infect Dis. 2018. PMID: 29976518 Free PMC article. No abstract available.
Abstract
Background: Pneumococcal conjugate vaccines (PCVs) are used in many low-income countries but their impact on the incidence of pneumonia is unclear. The Gambia introduced PCV7 in August, 2009, and PCV13 in May, 2011. We aimed to measure the impact of the introduction of these vaccines on pneumonia incidence.
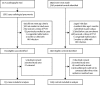
Methods: We did population-based surveillance and case-control studies. The primary endpoint was WHO-defined radiological pneumonia with pulmonary consolidation. Population-based surveillance was for suspected pneumonia in children aged 2-59 months (minimum age 3 months in the case-control study) between May 12, 2008, and Dec 31, 2015. Surveillance for the impact study was limited to the Basse Health and Demographic Surveillance System (BHDSS), whereas surveillance for the case-control study included both the BHDSS and Fuladu West Health and Demographic Surveillance System. Nurses screened all outpatients and inpatients at all health facilities in the surveillance area using standardised criteria for referral to clinicians in Basse and Bansang. These clinicians recorded clinical findings and applied standardised criteria to identify patients with suspected pneumonia. We compared the incidence of pneumonia during the baseline period (May 12, 2008, to May 11, 2010) and the PCV13 period (Jan 1, 2014, to Dec 31, 2015). We also investigated the effectiveness of PCV13 using case-control methods between Sept 12, 2011, and Sept 31, 2014. Controls were aged 90 days or older, and were eligible to have received at least one dose of PCV13; cases had the same eligibility criteria with the addition of having WHO-defined radiological pneumonia.
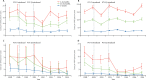
Findings: We investigated 18 833 children with clinical pneumonia and identified 2156 cases of radiological pneumonia. Among children aged 2-11 months, the incidence of radiological pneumonia fell from 21·0 cases per 1000 person-years in the baseline period to 16·2 cases per 1000 person-years (23% decline, 95% CI 7-36) in 2014-15. In the 12-23 month age group, radiological pneumonia decreased from 15·3 to 10·9 cases per 1000 person-years (29% decline, 12-42). In children aged 2-4 years, incidence fell from 5·2 to 4·1 cases per 1000 person-years (22% decline, 1-39). Incidence of all clinical pneumonia increased by 4% (-1 to 8), but hospitalised cases declined by 8% (3-13). Pneumococcal pneumonia declined from 2·9 to 1·2 cases per 1000 person-years (58% decline, 22-77) in children aged 2-11 months and from 2·6 to 0·7 cases per 1000 person-years (75% decline, 47-88) in children aged 12-23 months. Hypoxic pneumonia fell from 13·1 to 5·7 cases per 1000 person-years (57% decline, 42-67) in children aged 2-11 months and from 6·8 to 1·9 cases per 1000 person-years (72% decline, 58-82) in children aged 12-23 months. In the case-control study, the best estimate of the effectiveness of three doses of PCV13 against radiological pneumonia was an adjusted odds ratio of 0·57 (0·30-1·08) in children aged 3-11 months and vaccine effectiveness increased with greater numbers of doses (p=0·026). The analysis in children aged 12 months and older was underpowered because there were few unvaccinated cases and controls.
Interpretation: The introduction of PCV in The Gambia was associated with a moderate impact on the incidence of radiological pneumonia, a small reduction in cases of hospitalised pneumonia, and substantial reductions of pneumococcal and hypoxic pneumonia in young children. Low-income countries that introduce PCV13 with reasonable coverage can expect modest reductions in hospitalised cases of pneumonia and a marked impact on the incidence of severe childhood pneumonia.
Funding: GAVI's Pneumococcal vaccines Accelerated Development and Introduction Plan, Bill & Melinda Gates Foundation, and UK Medical Research Council.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Filling evidence gaps on the impact of pneumococcal vaccines.Lancet Infect Dis. 2017 Sep;17(9):888-889. doi: 10.1016/S1473-3099(17)30328-6. Epub 2017 Jun 7. Lancet Infect Dis. 2017. PMID: 28601420 Free PMC article. No abstract available.
References
-
- Liu L, Oza S, Hogan D. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. - PubMed
-
- Shann F, Gratten M, Germer S, Linnemann V, Hazlett D, Payne R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet. 1984;2:537–541. - PubMed
-
- Falade AG, Mulholland EK, Adegbola RA, Greenwood BM. Bacterial isolates from blood and lung aspirate cultures in Gambian children with lobar pneumonia. Ann Trop Paediatr. 1997;17:315–319. - PubMed
-
- O'Brien KL, Wolfson LJ, Watt JP. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

