Heterogeneous Cadherin Expression and Multicellular Aggregate Dynamics in Ovarian Cancer Dissemination
- PMID: 28601643
- PMCID: PMC5497527
- DOI: 10.1016/j.neo.2017.04.002
Heterogeneous Cadherin Expression and Multicellular Aggregate Dynamics in Ovarian Cancer Dissemination
Abstract
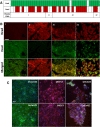
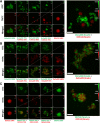
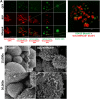
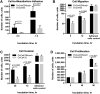
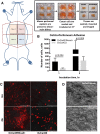
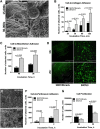
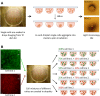
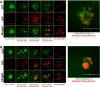
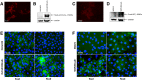
Epithelial ovarian carcinoma spreads via shedding of cells and multicellular aggregates (MCAs) from the primary tumor into peritoneal cavity, with subsequent intraperitoneal tumor cell:mesothelial cell adhesion as a key early event in metastatic seeding. Evaluation of human tumor extracts and tissues confirms that well-differentiated ovarian tumors express abundant E-cadherin (Ecad), whereas advanced lesions exhibit upregulated N-cadherin (Ncad). Two expression patterns are observed: "mixed cadherin," in which distinct cells within the same tumor express either E- or Ncad, and "hybrid cadherin," wherein single tumor cell(s) simultaneously expresses both cadherins. We demonstrate striking cadherin-dependent differences in cell-cell interactions, MCA formation, and aggregate ultrastructure. Mesenchymal-type Ncad+ cells formed stable, highly cohesive solid spheroids, whereas Ecad+ epithelial-type cells generated loosely adhesive cell clusters covered by uniform microvilli. Generation of "mixed cadherin" MCAs using fluorescently tagged cell populations revealed preferential sorting into cadherin-dependent clusters, whereas mixing of cell lines with common cadherin profiles generated homogeneous aggregates. Recapitulation of the "hybrid cadherin" Ecad+/Ncad+ phenotype, via insertion of the CDH2 gene into Ecad+ cells, resulted in the ability to form heterogeneous clusters with Ncad+ cells, significantly enhanced adhesion to organotypic mesomimetic cultures and peritoneal explants, and increased both migration and matrix invasion. Alternatively, insertion of CDH1 gene into Ncad+ cells greatly reduced cell-to-collagen, cell-to-mesothelium, and cell-to-peritoneum adhesion. Acquisition of the hybrid cadherin phenotype resulted in altered MCA surface morphology with increased surface projections and increased cell proliferation. Overall, these findings support the hypothesis that MCA cadherin composition impacts intraperitoneal cell and MCA dynamics and thereby affects ultimate metastatic success.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


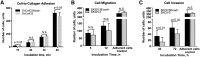
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. - PubMed
-
- Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse S, Kosary C, Ruhl J, Tatalovich Z. National Cancer Institute; Bethesda, MD: 2011. SEER cancer statistics review, 1975-2008; p. 19.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

