Microsomal triglyceride transfer protein in the ectoparasitic crustacean salmon louse (Lepeophtheirus salmonis)
- PMID: 28601811
- PMCID: PMC5538283
- DOI: 10.1194/jlr.M076430
Microsomal triglyceride transfer protein in the ectoparasitic crustacean salmon louse (Lepeophtheirus salmonis)
Abstract
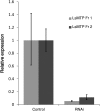
The salmon louse, Lepeophtheirus salmonis, is an endemic ectoparasite on salmonid fish that is challenging for the salmon farming industry and wild fish. Salmon lice produce high numbers of offspring, necessitating sequestration of large amounts of lipids into growing oocytes as a major energy source for larvae, most probably mediated by lipoproteins. The microsomal triglyceride transfer protein (MTP) is essential for the assembly of lipoproteins. Salmon lice have three L. salmonis MTP (LsMTP) transcript variants encoding two different protein isoforms, which are predicted to contain three β-sheets (N, C, and A) and a central helical domain, similar to MTPs from other species. In adult females, the LsMTPs are differently transcribed in the sub-cuticular tissues, the intestine, the ovary, and in the mature eggs. RNA interference-mediated knockdown of LsMTP in mature females gave offspring with significantly fewer neutral lipids in their yolk and only 10-30% survival. The present study suggests the importance of LsMTP in reproduction and lipid metabolism in adult female L. salmonis, a possible metabolic bottleneck that could be exploited for the development of new anti-parasitic treatment methods.
Keywords: Nile Red; Oil Red O; RNA interference; gene expression; lipid and lipoprotein metabolism; lipid transport; lipid transport proteins; lipoproteins; sea lice.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Molecular characterization of the lipophorin receptor in the crustacean ectoparasite Lepeophtheirus salmonis.PLoS One. 2018 Apr 12;13(4):e0195783. doi: 10.1371/journal.pone.0195783. eCollection 2018. PLoS One. 2018. PMID: 29649335 Free PMC article.
-
Characterisation of iron regulatory protein 1A and 1B in the blood-feeding copepod Lepeophtheirus salmonis.Exp Parasitol. 2015 Oct;157:1-11. doi: 10.1016/j.exppara.2015.06.010. Epub 2015 Jun 24. Exp Parasitol. 2015. PMID: 26115940
-
The ecdysone receptor (EcR) is a major regulator of tissue development and growth in the marine salmonid ectoparasite, Lepeophtheirus salmonis (Copepoda, Caligidae).Mol Biochem Parasitol. 2016 Aug;208(2):65-73. doi: 10.1016/j.molbiopara.2016.06.007. Epub 2016 Jun 21. Mol Biochem Parasitol. 2016. PMID: 27345580
-
Analysis and management of resistance to chemotherapeutants in salmon lice, Lepeophtheirus salmonis (Copepoda: Caligidae).Pest Manag Sci. 2002 Jun;58(6):528-36. doi: 10.1002/ps.482. Pest Manag Sci. 2002. PMID: 12138619 Review.
-
Physiology and immunology of Lepeophtheirus salmonis infections of salmonids.Trends Parasitol. 2008 Apr;24(4):176-83. doi: 10.1016/j.pt.2007.12.010. Epub 2008 Mar 7. Trends Parasitol. 2008. PMID: 18329341 Review.
Cited by
-
Two apolipoproteins in salmon louse (Lepeophtheirus salmonis), apolipoprotein 1 knock down reduces reproductive capacity.Biochem Biophys Rep. 2021 Oct 22;28:101156. doi: 10.1016/j.bbrep.2021.101156. eCollection 2021 Dec. Biochem Biophys Rep. 2021. PMID: 34729423 Free PMC article.
-
Host gill attachment causes blood-feeding by the salmon louse (Lepeophtheirus salmonis) chalimus larvae and alters parasite development and transcriptome.Parasit Vectors. 2020 May 6;13(1):225. doi: 10.1186/s13071-020-04096-0. Parasit Vectors. 2020. PMID: 32375890 Free PMC article.
-
dLp/HDL-BGBP and MTP Cloning and Expression Profiles During Embryonic Development in the Mud Crab Scylla paramamosain.Front Physiol. 2021 Aug 19;12:717751. doi: 10.3389/fphys.2021.717751. eCollection 2021. Front Physiol. 2021. PMID: 34489734 Free PMC article.
-
Molecular characterization of the lipophorin receptor in the crustacean ectoparasite Lepeophtheirus salmonis.PLoS One. 2018 Apr 12;13(4):e0195783. doi: 10.1371/journal.pone.0195783. eCollection 2018. PLoS One. 2018. PMID: 29649335 Free PMC article.
-
Polar and neutral lipid composition of the copepod Lernaeocera lusci and its host Merluccius merluccius in relationship with the parasite intensity.Parasitol Res. 2021 Jun;120(6):1979-1991. doi: 10.1007/s00436-021-07182-z. Epub 2021 May 14. Parasitol Res. 2021. PMID: 33987737
References
-
- Wetterau J. R., and Zilversmit D. B.. 1984. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J. Biol. Chem. 259: 10863–10866. - PubMed
-
- Wetterau J. R., Lin M. C., and Jamil H.. 1997. Microsomal triglyceride transfer protein. Biochim. Biophys. Acta. 1345: 136–150. - PubMed
-
- Jamil H., Dickson J. K. Jr., Chu C. H., Lago M. W., Rinehart J. K., Biller S. A., Gregg R. E., and Wetterau J. R.. 1995. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J. Biol. Chem. 270: 6549–6554. - PubMed
-
- Babin P. J., Bogerd J., Kooiman F. P., Van Marrewijk W. J., and Van der Horst D. J.. 1999. Apolipophorin II/I, apolipoprotein B, vitellogenin, and microsomal triglyceride transfer protein genes are derived from a common ancestor. J. Mol. Evol. 49: 150–160. - PubMed
-
- Wu L. T., Hui J. H., and Chu K. H.. 2013. Origin and evolution of yolk proteins: expansion and functional diversification of large lipid transfer protein superfamily. Biol. Reprod. 88: 102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

