Targeted simplification versus antipseudomonal broad-spectrum beta-lactams in patients with bloodstream infections due to Enterobacteriaceae (SIMPLIFY): a study protocol for a multicentre, open-label, phase III randomised, controlled, non-inferiority clinical trial
- PMID: 28601833
- PMCID: PMC5734512
- DOI: 10.1136/bmjopen-2016-015439
Targeted simplification versus antipseudomonal broad-spectrum beta-lactams in patients with bloodstream infections due to Enterobacteriaceae (SIMPLIFY): a study protocol for a multicentre, open-label, phase III randomised, controlled, non-inferiority clinical trial
Abstract
Introduction: Within the context of antimicrobial stewardship programmes, de-escalation of antimicrobial therapy is one of the proposed strategies for reducing the unnecessary use of broad-spectrum antibiotics (BSA). The empirical treatment of nosocomial and some healthcare-associated bloodstream infections (BSI) frequently includes a beta-lactam with antipseudomonal activity as monotherapy or in combination with other drugs, so there is a great opportunity to optimise the empirical therapy based on microbiological data. De-escalation is assumed as standard of care for experts in infectious diseases. However, it is less frequent than it would desirable.
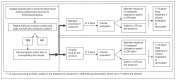
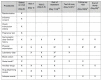
Methods and analysis: The SIMPLIFY trial is a multicentre, open-label, non-inferiority phase III randomised controlled clinical trial, designed as a pragmatic 'real-practice' trial. The aim of this trial is to demonstrate the non-inferiority of de-escalation from an empirical beta-lactam with antipseudomonal activity to a targeted narrow-spectrum antimicrobial in patients with BSI due to Enterobacteriaceae. The primary outcome is clinical cure, which will be assessed at the test of cure visit. It will be conducted at 19 Spanish public and university hospitals.
Ethics and dissemination: Each participating centre has obtained the approval of the ethics review committee, the agreement of the directors of the institutions and authorisation from the Spanish Regulatory Agency (Agencia Española del Medicamento y Productos Sanitarios). Data will be presented at international conferences and published in peer-reviewed journals.
Discussion: Strategies to reduce the use of BSA should be a priority. Most of the studies that support de-escalation are observational, retrospective and heterogeneous. A recent Cochrane review stated that well-designed clinical trials should be conducted to assess the safety and efficacy of de-escalation.
Trial registration number: The European Union Clinical Trials Register: EudraCT number 2015-004219-19. Clinical trials.gov: NCT02795949. Protocol version: V.2.0, dated 16 May 2016. All items from the WHO Trial Registration Data Set are included in the registry.
Keywords: Enterobacteriaceae; De-escalation; antimicrobial stewardship; bloodstream infection; broad-spectrum antibiotics.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Rodríguez-Baño J, Paño-Pardo JR, Álvarez-Rocha L, et al. . Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Enferm Infecc Microbiol Clin 2012;30:22.e1–22.e23. - PubMed
-
- Dellit TH, Owens RC, McGowan JE, et al. . Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–77. - PubMed
-
- Rodríguez-Baño J, López-Prieto MD, Portillo MM, et al. . Epidemiology and clinical features of community-acquired, healthcare-associated and nosocomial bloodstream infections in tertiary-care and community hospitals. Clin Microbiol Infect 2010;16:1408–13. 10.1111/j.1469-0691.2010.03089.x - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical