Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes
- PMID: 28602020
- PMCID: PMC6481528
- DOI: 10.1002/14651858.CD009613.pub3
Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes
Update in
-
Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes.Cochrane Database Syst Rev. 2019 May 23;5(5):CD009613. doi: 10.1002/14651858.CD009613.pub4. Cochrane Database Syst Rev. 2019. PMID: 31120549 Free PMC article.
Abstract
Background: Self-monitoring of blood glucose (SMBG) is recommended as a key component of the management plan for diabetes therapy during pregnancy. No existing systematic reviews consider the benefits/effectiveness of various techniques of blood glucose monitoring on maternal and infant outcomes among pregnant women with pre-existing diabetes. The effectiveness of the various monitoring techniques is unclear.
Objectives: To compare techniques of blood glucose monitoring and their impact on maternal and infant outcomes among pregnant women with pre-existing diabetes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 November 2016), searched reference lists of retrieved studies and contacted trial authors.
Selection criteria: Randomised controlled trials (RCTs) and quasi-RCTs comparing techniques of blood glucose monitoring including SMBG, continuous glucose monitoring (CGM) or clinic monitoring among pregnant women with pre-existing diabetes mellitus (type 1 or type 2). Trials investigating timing and frequency of monitoring were also included. RCTs using a cluster-randomised design were eligible for inclusion but none were identified.
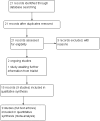
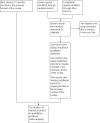
Data collection and analysis: Two review authors independently assessed study eligibility, extracted data and assessed the risk of bias of included studies. Data were checked for accuracy. The quality of the evidence was assessed using the GRADE approach.
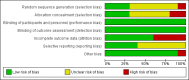
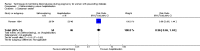
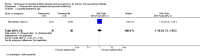
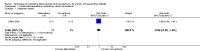
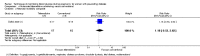
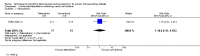
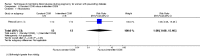
Main results: This review update includes at total of 10 trials (538) women (468 women with type 1 diabetes and 70 women with type 2 diabetes). The trials took place in Europe and the USA. Five of the 10 included studies were at moderate risk of bias, four studies were at low to moderate risk of bias, and one study was at high risk of bias. The trials are too small to show differences in important outcomes such as macrosomia, preterm birth, miscarriage or death of baby. Almost all the reported GRADE outcomes were assessed as being very low-quality evidence. This was due to design limitations in the studies, wide confidence intervals, small sample sizes, and few events. In addition, there was high heterogeneity for some outcomes.Various methods of glucose monitoring were compared in the trials. Neither pooled analyses nor individual trial analyses showed any clear advantages of one monitoring technique over another for primary and secondary outcomes. Many important outcomes were not reported.1. Self-monitoring versus standard care (two studies, 43 women): there was no clear difference for caesarean section (risk ratio (RR) 0.78, 95% confidence interval (CI) 0.40 to 1.49; one study, 28 women) or glycaemic control (both very low-quality), and not enough evidence to assess perinatal mortality and neonatal mortality and morbidity composite. Hypertensive disorders of pregnancy, large-for-gestational age, neurosensory disability, and preterm birth were not reported in either study.2. Self-monitoring versus hospitalisation (one study, 100 women): there was no clear difference for hypertensive disorders of pregnancy (pre-eclampsia and hypertension) (RR 4.26, 95% CI 0.52 to 35.16; very low-quality: RR 0.43, 95% CI 0.08 to 2.22; very low-quality). There was no clear difference in caesarean section or preterm birth less than 37 weeks' gestation (both very low quality), and the sample size was too small to assess perinatal mortality (very low-quality). Large-for-gestational age, mortality or morbidity composite, neurosensory disability and preterm birth less than 34 weeks were not reported.3. Pre-prandial versus post-prandial glucose monitoring (one study, 61 women): there was no clear difference between groups for caesarean section (RR 1.45, 95% CI 0.92 to 2.28; very low-quality), large-for-gestational age (RR 1.16, 95% CI 0.73 to 1.85; very low-quality) or glycaemic control (very low-quality). The results for hypertensive disorders of pregnancy: pre-eclampsia and perinatal mortality are not meaningful because these outcomes were too rare to show differences in a small sample (all very low-quality). The study did not report the outcomes mortality or morbidity composite, neurosensory disability or preterm birth.4. Automated telemedicine monitoring versus conventional system (three studies, 84 women): there was no clear difference for caesarean section (RR 0.96, 95% CI 0.62 to 1.48; one study, 32 women; very low-quality), and mortality or morbidity composite in the one study that reported these outcomes. There were no clear differences for glycaemic control (very low-quality). No studies reported hypertensive disorders of pregnancy, large-for-gestational age, perinatal mortality (stillbirth and neonatal mortality), neurosensory disability or preterm birth.5.CGM versus intermittent monitoring (two studies, 225 women): there was no clear difference for pre-eclampsia (RR 1.37, 95% CI 0.52 to 3.59; low-quality), caesarean section (average RR 1.00, 95% CI 0.65 to 1.54; I² = 62%; very low-quality) and large-for-gestational age (average RR 0.89, 95% CI 0.41 to 1.92; I² = 82%; very low-quality). Glycaemic control indicated by mean maternal HbA1c was lower for women in the continuous monitoring group (mean difference (MD) -0.60 %, 95% CI -0.91 to -0.29; one study, 71 women; moderate-quality). There was not enough evidence to assess perinatal mortality and there were no clear differences for preterm birth less than 37 weeks' gestation (low-quality). Mortality or morbidity composite, neurosensory disability and preterm birth less than 34 weeks were not reported.6. Constant CGM versus intermittent CGM (one study, 25 women): there was no clear difference between groups for caesarean section (RR 0.77, 95% CI 0.33 to 1.79; very low-quality), glycaemic control (mean blood glucose in the 3rd trimester) (MD -0.14 mmol/L, 95% CI -2.00 to 1.72; very low-quality) or preterm birth less than 37 weeks' gestation (RR 1.08, 95% CI 0.08 to 15.46; very low-quality). Other primary (hypertensive disorders of pregnancy, large-for-gestational age, perinatal mortality (stillbirth and neonatal mortality), mortality or morbidity composite, and neurosensory disability) or GRADE outcomes (preterm birth less than 34 weeks' gestation) were not reported.
Authors' conclusions: This review found no evidence that any glucose monitoring technique is superior to any other technique among pregnant women with pre-existing type 1 or type 2 diabetes. The evidence base for the effectiveness of monitoring techniques is weak and additional evidence from large well-designed randomised trials is required to inform choices of glucose monitoring techniques.
Conflict of interest statement
Foong Ming Moy: none declared.
Amita Ray: none declared.
Brian Buckley: none declared.
Helen West: was paid to work on Cochrane reviews by a grant to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Figures



















































































Update of
-
Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes.Cochrane Database Syst Rev. 2014 Apr 30;(4):CD009613. doi: 10.1002/14651858.CD009613.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Jun 11;6:CD009613. doi: 10.1002/14651858.CD009613.pub3. PMID: 24782359 Updated.
Similar articles
-
Techniques of monitoring blood glucose during pregnancy for women with pre-existing diabetes.Cochrane Database Syst Rev. 2014 Apr 30;(4):CD009613. doi: 10.1002/14651858.CD009613.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Jun 11;6:CD009613. doi: 10.1002/14651858.CD009613.pub3. PMID: 24782359 Updated.
-
Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus.Cochrane Database Syst Rev. 2017 Jan 3;1(1):CD006674. doi: 10.1002/14651858.CD006674.pub3. Cochrane Database Syst Rev. 2017. PMID: 28046205 Free PMC article.
-
Intermittent auscultation (IA) of fetal heart rate in labour for fetal well-being.Cochrane Database Syst Rev. 2017 Feb 13;2(2):CD008680. doi: 10.1002/14651858.CD008680.pub2. Cochrane Database Syst Rev. 2017. PMID: 28191626 Free PMC article.
-
Continuous subcutaneous insulin infusion versus multiple daily injections of insulin for pregnant women with diabetes.Cochrane Database Syst Rev. 2016 Jun 7;2016(6):CD005542. doi: 10.1002/14651858.CD005542.pub3. Cochrane Database Syst Rev. 2016. PMID: 27272351 Free PMC article.
-
Different methods and settings for glucose monitoring for gestational diabetes during pregnancy.Cochrane Database Syst Rev. 2017 Oct 29;10(10):CD011069. doi: 10.1002/14651858.CD011069.pub2. Cochrane Database Syst Rev. 2017. PMID: 29081069 Free PMC article.
Cited by
-
Telemedicine and Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis.Cureus. 2024 Oct 20;16(10):e71907. doi: 10.7759/cureus.71907. eCollection 2024 Oct. Cureus. 2024. PMID: 39564055 Free PMC article. Review.
-
Emerging Technologies for the Management of Type 1 Diabetes in Pregnancy.Curr Diab Rep. 2018 Jan 30;18(1):4. doi: 10.1007/s11892-018-0973-9. Curr Diab Rep. 2018. PMID: 29383544 Free PMC article. Review.
-
Telemedicine in Complex Diabetes Management.Curr Diab Rep. 2018 May 24;18(7):42. doi: 10.1007/s11892-018-1015-3. Curr Diab Rep. 2018. PMID: 29797292 Review.
-
Correlates of blood pressure and blood glucose screenings in Cameroon: insights from the 2018 Demographic and Health Survey.Int Health. 2022 Mar 2;14(2):201-210. doi: 10.1093/inthealth/ihab033. Int Health. 2022. PMID: 34118153 Free PMC article.
-
Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews.Cochrane Database Syst Rev. 2018 Nov 14;11(11):CD012505. doi: 10.1002/14651858.CD012505.pub2. Cochrane Database Syst Rev. 2018. PMID: 30480756 Free PMC article.
References
References to studies included in this review
-
- Dalfrà MG, Nicolucci A, Lapolla A, TISG. The effect of telemedicine on outcome and quality of life in pregnant women with diabetes. Journal of Telemedicine & Telecare 2009;15(5):238‐42. - PubMed
-
- Biase N, Napoli A, Sabbatini A, Borrello E, Buongiorno AM, Fallucca F. Telemedicine in the treatment of diabetic pregnancy. Annali dell Istituto Superiore di Sanita 1997;33:347‐51. - PubMed
-
- Hanson U, Persson B, Enochsson E, Lennerhagen P, Lindgren F, Lundstrom V, et al. Self‐monitoring of blood glucose by diabetic women during the third trimester of pregnancy. American Journal of Obstetrics and Gynecology 1984;150:817‐21. - PubMed
-
- Manderson J, Ennis C, Patterson C, Hadden D, Traub A. Pre‐eclampsia in type 1 diabetic pregnancy: preprandial versus postprandial capillary blood glucose monitoring. Hypertension in Pregnancy 2002;21(Suppl 1):142.
- Manderson JG, Patterson CC, Hadden DR, Traub AI, Ennis C, McCance DR. Preprandial versus postprandial blood glucose monitoring in type 1 diabetic pregnancy: a randomized controlled clinical trial. American Journal of Obstetrics and Gynecology 2003;189:507‐12. - PubMed
-
- ISRCTN84461581. A randomised controlled trial to evaluate the role of the continuous glucose monitoring system (CGMS) in pregnancies complicated by pre‐existing diabetes. isrctn.com/ISRCTN84461581 Date first received: 30 September 2005.
- Murphy HR, Rayman G, Duffield K, Lewis KS, Kelly S, Johal B, et al. Changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. Diabetes Care 2007;30(11):2785‐91. - PubMed
- Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. BMJ 2008;337:a1680. - PMC - PubMed
References to studies excluded from this review
-
- Bartholomew ML, Church K, Graham G, Burlingame J, Zalud I, Sauvage L, et al. Managing diabetes in pregnancy using cell phone/internet technology. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S113‐S114. - PubMed
- NCT01907516. Managing diabetes in pregnancy using cell phone/internet technology. clinicaltrials.gov/show/NCT01907516 Date first received: 22 July 2013.
-
- NCT01630759. Remote monitoring of diabetes in pregnancy: a feasibility study for a randomised controlled trial. clinicaltrials.gov/show/NCT01630759 Date first received: 22 June 2012.
-
- Temple RC, Duffield K, Lewis K, Murphy HR. Glycaemic control during pregnancy in women with long duration type 1 diabetes: lessons learn using continuous glucose monitoring systems. Diabetologia 2006;49(Suppl 1):S78.
-
- Walker JD. Blood glucose monitoring strategies in diabetic: an audit of achievement of glycaemic goals and outcome of pregnancy. National Research Register (www.nrr.nhs.uk)1999.
References to ongoing studies
-
- Farrell A, Mergler S, Mason D, Sanchez J, Feig DS, Asztalos E. The use of logs and forms for the tracking of RT‐CGM devices in the CONCEPTT Trial. Clinical Trials 2013;10:S80.
- Feig DS, Asztalos E, Corcoy R, Leiva A, Donovan L, Hod M, et al. CONCEPTT: Continuous Glucose Monitoring in Women with Type 1 Diabetes in Pregnancy Trial: A multi‐center, multi‐national, randomized controlled trial ‐ Study protocol. BMC Pregnancy and Childbirth 2016;16(1):167. [PUBMED: 27430714] - PMC - PubMed
- NCT01788527. Continuous glucose monitoring in women with type 1 diabetes in pregnancy trial (CONCEPTT). clinicaltrials.gov/show/NCT01788527 Date first received: 19 December 2012.
-
- Evers I. Effectiveness of continuous glucose monitoring during diabetic pregnancy (GlucoMOMS trial); a randomised controlled trial. Diabetes Technology and Therapeutics 2016;18:A13‐A14. - PMC - PubMed
- Voormolen DN, DeVries JH, Franx A, Mol BW, Evers IM. Effectiveness of continuous glucose monitoring during diabetic pregnancy (GlucoMOMS trial); a randomised controlled trial. BMC Pregnancy and Childbirth 2012;12:164. - PMC - PubMed
Additional references
-
- ACOG Committee on Practice Bulletins, authors. Pregestational diabetes mellitus: ACOG Clinical Management Guidelines for Obstetrician‐Gynecologists #60. Obstetrics & Gynecology 2005;105:675‐85. - PubMed
-
- ADA. Preconception care of women with diabetes (Position Statement). Diabetes Care 2004;27:S76‐S78. - PubMed
-
- Choleau C, Klein JC, Reach G, Aussedat B, Demaria‐Pesce V, Wilson GS, et al. Calibration of a subcutaneous amperometric glucose sensor. Part 1. Effect of measurement uncertainties on the determination of sensor sensitivity and background current. Biosensors and Bioelectronics 2002;17(8):641–6. - PubMed
-
- Davidson J. Strategies for improving glycemic control: effective use of glucose monitoring. American Journal of Medicine 2005;118(9 Suppl 1):27–32. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

