Secukinumab demonstrates greater sustained improvements in daily activities and personal relationships than ustekinumab in patients with moderate-to-severe plaque psoriasis: 52-week results from the CLEAR study
- PMID: 28602039
- PMCID: PMC6084293
- DOI: 10.1111/jdv.14391
Secukinumab demonstrates greater sustained improvements in daily activities and personal relationships than ustekinumab in patients with moderate-to-severe plaque psoriasis: 52-week results from the CLEAR study
Abstract
Background: Psoriasis can greatly impact patients' lives by influencing clothing worn as well as by impairing sexual functioning. Secukinumab, a human monoclonal antibody selectively neutralizing interleukin-17A, has demonstrated good efficacy and safety in the treatment of moderate-to-severe psoriasis and psoriatic arthritis with a rapid onset of action and sustained response.
Objective: This analysis using the CLEAR study, a phase 3b double-blind study comparing the efficacy and safety of secukinumab vs. ustekinumab in adults with moderate-to-severe plaque psoriasis, evaluated the treatment effects on patient's daily activities and personal relationships.
Methods: Impact on daily activities (interference with home/shopping/garden, and influence on clothes worn) and impact on personal relationships (problems with partner/others, and sexual difficulties) as well as their corresponding subscales were selected from the Dermatology Life Quality Index scale and evaluated for patients treated with secukinumab vs. ustekinumab from the CLEAR study. Treatment differences in mean scores and proportions of responders (score = 0, indicating no impact) were evaluated through 52 weeks. Time to response was evaluated through Week 16.
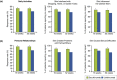
Results: Significant differences between secukinumab and ustekinumab were observed for daily activities and personal relationships at Week 16 and sustained through Week 52 (Week 52 response rates for daily activities: 82.9% vs. 73.5%, including interference with home/shopping/garden: 88.5% vs. 78.2%, and influence on clothes worn: 85.6% vs. 74.4%; personal relationships: 86.1% vs. 73.7%, including problems with partner/others: 86.6% vs. 74.8%, and sexual difficulties: 88.5% vs. 74.3%; all P < 0.01). The median time to response was 4 weeks for secukinumab vs. 8 weeks for ustekinumab for daily activities and personal relationships (both P < 0.05).
Conclusion: Secukinumab treatment helps patients with moderate-to-severe plaque psoriasis have a more normal life faster when compared to ustekinumab, by providing greater and sustained improvement in clothing choice and sexual functioning.
© 2017 European Academy of Dermatology and Venereology.
Figures

References
-
- Lebwohl M, Swensen AR, Nyirady J et al The Psoriasis Symptom Diary: development and content validity of a novel patient‐reported outcome instrument. Int J Dermatol 2014; 53: 714–722. - PubMed
-
- Lebwohl MG, Bachelez H, Barker J et al Patient perspectives in the management of psoriasis: results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol 2014; 70: 871–881. - PubMed
-
- de Korte J, Sprangers MA, Mombers FM et al Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc 2004; 9: 140–147. - PubMed
-
- Blome C, Gosau R, Radtke MA et al Patient‐relevant treatment goals in psoriasis. Arch Dermatol Res 2016; 308: 69–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

