CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma
- PMID: 28602436
- PMCID: PMC5589058
- DOI: 10.1016/j.ymthe.2017.05.012
CAR T Cells Administered in Combination with Lymphodepletion and PD-1 Inhibition to Patients with Neuroblastoma
Abstract
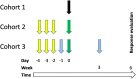
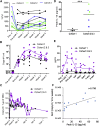
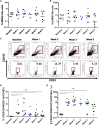
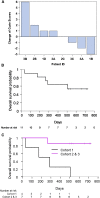
Targeting disialoganglioside (GD2) on neuroblastoma (NB) with T cells expressing a first-generation chimeric antigen receptor (CAR) was safe, but the cells had poor expansion and long-term persistence. We developed a third-generation GD2-CAR (GD2-CAR3) and hypothesized that GD2-CAR3 T cells (CARTs) would be safe and effective. This phase 1 study enrolled relapsed or refractory NB patients in three cohorts. Cohort 1 received CART alone, cohort 2 received CARTs plus cyclophosphamide and fludarabine (Cy/Flu), and cohort 3 was treated with CARTs, Cy/Flu, and a programmed death-1 (PD-1) inhibitor. Eleven patients were treated with CARTs. The infusions were safe, and no dose-limiting toxicities occurred. CARTs were detectable in cohort 1, but the lymphodepletion induced by Cy/Flu increased circulating levels of the homeostatic cytokine interleukin (IL)-15 (p = 0.003) and increased CART expansion by up to 3 logs (p = 0.03). PD-1 inhibition did not further enhance expansion or persistence. Antitumor responses at 6 weeks were modest. We observed a striking expansion of CD45/CD33/CD11b/CD163+ myeloid cells (change from baseline, p = 0.0126) in all patients, which may have contributed to the modest early antitumor responses; the effect of these cells merits further study. Thus, CARTs are safe, and Cy/Flu can further increase their expansion.
Keywords: GD2-CAR; immunotherapy; neuroblastoma.
Copyright © 2017 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Cheung N.K., Cheung I.Y., Kushner B.H., Ostrovnaya I., Chamberlain E., Kramer K., Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J. Clin. Oncol. 2012;30:3264–3270. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

