Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study
- PMID: 28602585
- PMCID: PMC8207822
- DOI: 10.1016/S2352-3026(17)30088-1
Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study
Abstract
Background: Myelofibrosis is a chronic myeloproliferative neoplasm characterised by splenomegaly, cytopenias, bone marrow fibrosis, and debilitating symptoms including fatigue, weight loss, and bone pain. Mutations in Janus kinase-2 (JAK2) occur in approximately 50% of patients. The only approved JAK2 inhibitor for myelofibrosis is the dual JAK1 and JAK2 inhibitor, ruxolitinib. 58-71% of patients treated with ruxolitinib in clinical trials so far have not achieved the primary endpoint of 35% or more reduction in spleen volume from baseline assessed by MRI or CT. Furthermore, more than 50% of patients discontinue ruxolitinib treatment after 3-5 years. On the basis of this unmet need, we investigated the efficacy and safety of fedratinib, a JAK2-selective inhibitor, in patients with ruxolitinib-resistant or ruxolitinib-intolerant myelofibrosis.
Methods: This single-arm, open-label, non-randomised, phase 2, multicentre study, done at 31 sites in nine countries, enrolled adult patients with a current diagnosis of intermediate or high-risk primary myelofibrosis, post-polycythaemia vera myelofibrosis, or post-essential thrombocythaemia myelofibrosis, found to be ruxolitinib resistant or intolerant after at least 14 days of treatment. Other main inclusion criteria were palpable splenomegaly (≥5 cm below the left costal margin), Eastern Cooperative Oncology Group performance status of 2 or less, and life expectancy of 6 months or less. Patients received oral fedratinib at a starting dose of 400 mg once per day, for six consecutive 28-day cycles. The primary endpoint was spleen response (defined as the proportion of patients with a ≥35% reduction in spleen volume as determined by blinded CT and MRI at a central imaging laboratory). We did the primary analysis in the per-protocol population only (patients treated with fedratinib, for whom a baseline and at least one post-baseline spleen volume measurement was available) and the safety analysis in all patients receiving at least one dose of fedratinib. This trial was registered with ClinicalTrials.gov, number NCT01523171.
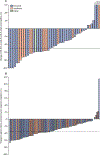
Findings: Between May 8, 2012, and Aug 29, 2013, 97 patients were enrolled and received at least one dose of fedratinib. Of 83 assessable patients, 46 (55%, 95% CI 44-66) achieved a spleen response. Common grade 3-4 adverse events included anaemia (37 [38%] of 97 patients) and thrombocytopenia (21 [22%] of 97), with 18 (19%) patients discontinuing due to adverse events. Seven (7%) patients died during the study, but none of the deaths was drug related. Suspected cases of Wernicke's encephalopathy in other fedratinib trials led to study termination.
Interpretation: This phase 2 study met its primary endpoint, suggesting that patients with ruxolitinib-resistant or ruxolitinib-intolerant myelofibrosis might achieve significant clinical benefit with fedratinib, albeit at the cost of some potential toxicity, which requires further evaluation. Fedratinib development in this setting is currently being assessed.
Funding: Sanofi.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CNH reports grants from Novartis and Shire; personal fees from Novartis, Sanofi, CTI, Baxalta, Gilead, and Shire; and other fees from Novartis, Sanofi, and CTI. AMV reports grants and personal fees from Novartis. J-JK reports personal fees and non-financial support from Novartis and Shire, and grants from AOP Orphan. RVT reports participation in advisory boards and speaker bureaus for Incyte and advisory meetings for Gilead Biosciences, and started employment at Eli Lilly and Company on Jan 19, 2015. EJ reports personal fees and non-financial support from Novartis. EW reports clinical trial grants from Sanofi-Aventis during the conduct of this study. HCS reports personal fees from Sanofi and Novartis. SZ reports grants for research and advisory board participation from Novartis. RAM reports grants from Sanofi, Incyte, Gilead, and CTI. RTS reports advisory board participation in AOP Orphan, PharmaEssentia, Incyte, and Gilead BioScience. MT reports research funds from Incyte, CTI Biopharma, NS Pharma, Gilead, and Sanofi; and advisory board participation for Sanofi, Gilead, CTI Biopharma, and Incyte. JL and ZS are employees of Sanofi. NS, PZ, and FP declare no competing interests.
Figures
Comment in
-
How to define treatment failure for JAK inhibitors.Lancet Haematol. 2017 Jul;4(7):e305-e306. doi: 10.1016/S2352-3026(17)30102-3. Epub 2017 Jun 8. Lancet Haematol. 2017. PMID: 28602584 No abstract available.
References
-
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. : JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: pp. 787–798. View In Article - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous