CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells
- PMID: 28602834
- PMCID: PMC5536847
- DOI: 10.1016/j.expneurol.2017.06.013
CNS disease diminishes the therapeutic functionality of bone marrow mesenchymal stem cells
Abstract
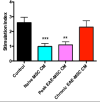
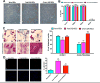
Mesenchymal stem cells (MSCs) have emerged as a potentially powerful cellular therapy for autoimmune diseases including multiple sclerosis (MS). Based on their success in treating animal models of MS like experimental autoimmune encephalomyelitis (EAE), MSCs have moved rapidly into clinical trials for MS. The majority of these trials use autologous MSCs derived from MS patients, although it remains unclear how CNS disease may affect these cells. Here, we report that bone marrow MSCs derived from EAE mice lack therapeutic efficacy compared to naïve MSCs in their ability to ameliorate EAE. Treatment with conditioned medium from EAE-MSCs also fails to modulate EAE, and EAE-MSCs secrete higher levels of many pro-inflammatory cytokines compared to naïve MSCs. Similarly, MSCs derived from MS patients have less therapeutic efficacy than naïve MSCs in treating EAE and secrete higher levels of some of the same pro-inflammatory cytokines. Thus diseases like EAE and MS diminish the therapeutic functionality of bone marrow MSCs, prompting reevaluation about the ongoing use of autologous MSCs as a treatment for MS.
Keywords: Experimental autoimmune encephalomyelitis; Mesenchymal stem cells (MSCs); Multiple sclerosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


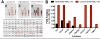

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

