The impact of VDR expression and regulation in vivo
- PMID: 28602960
- PMCID: PMC5723236
- DOI: 10.1016/j.jsbmb.2017.06.002
The impact of VDR expression and regulation in vivo
Abstract
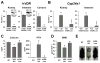
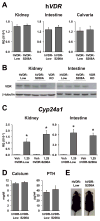
The vitamin D receptor (VDR) mediates the pleiotropic biological actions of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). These actions include orchestration of mineral homeostasis which is coordinated by the kidney, intestine, bone and parathyroid gland wherein the VDR transcriptionally regulates expression of the genes involved in this complex process. Mutations in human VDR (hVDR) cause hereditary vitamin D resistant rickets, a genetic syndrome characterized by hypocalcemia, hyperparathyroidism and rickets resulting from dysregulation of mineral homeostasis. Expression of the VDR is regulated by external stimuli in a tissue-specific manner. However, the mechanisms of this tissue-specificity remain unclear. Studies also suggest that phosphorylation of hVDR at serine 208 impacts the receptor's transcriptional activity. These experiments were conducted in vitro, however, and therefore limited in their conclusions. In this report, we summarize (1) our most recently updated ChIP-seq data from mouse tissues to identify regulatory regions responsible for the tissues-specific regulation of the VDR and (2) our studies to understand the mechanism of hormonal regulation of Vdr expression in bone and kidney in vivo using transgenic mouse strains generated by mouse mini-genes that contain comprehensive genetic information capable of recapitulating endogenous Vdr gene regulation and expression. We also defined the functional human VDR gene locus in vivo by using a human mini-gene comparable to that in the mouse to generate a humanized VDR mouse strain in which the receptor is expressed at normal levels (normal expressor). The present report also shows that a humanized mouse model in which the VDR is expressed at levels about 10-fold lower than the normal expressor mouse rescued the VDR-null phenotype despite its reduced transcriptional activity relative to wildtype expression. We also generated an additional humanized mouse model expressing hVDR bearing a mutation converting serine 208 to alanine (hVDR-S208A). In spite of the mutation, target gene expression induced by the ligand was unchanged relative to a mouse strain expressing comparable levels of wildtype hVDR. Further characterization also showed that serum calcium and parathyroid hormone levels were normal and alopecia was not observed in this hVDR-S208A mouse strain as well. Taken together, our in vivo studies using ChIP-seq analyses and the mini-gene transgenic mice improve our understanding of the tissue-specific regulatory mechanisms of controlling VDR expression and the mechanisms of action of the VDR.
Keywords: 1,25-dihydroxyvitamin D(3); Bacterial artificial chromosome; ChIP-seq; Hereditary vitamin D resistant rickets; Humanized VDR mouse model; Vitamin D receptor.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

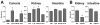


Similar articles
-
The vitamin D receptor functions as a transcription regulator in the absence of 1,25-dihydroxyvitamin D3.J Steroid Biochem Mol Biol. 2016 Nov;164:265-270. doi: 10.1016/j.jsbmb.2015.08.018. Epub 2015 Aug 29. J Steroid Biochem Mol Biol. 2016. PMID: 26323657 Free PMC article.
-
A humanized mouse model of hereditary 1,25-dihydroxyvitamin D-resistant rickets without alopecia.Endocrinology. 2014 Nov;155(11):4137-48. doi: 10.1210/en.2014-1417. Epub 2014 Aug 22. Endocrinology. 2014. PMID: 25147982 Free PMC article.
-
The vitamin D hormone and its nuclear receptor: molecular actions and disease states.J Endocrinol. 1997 Sep;154 Suppl:S57-73. J Endocrinol. 1997. PMID: 9379138 Review.
-
Transgenic Expression of the Vitamin D Receptor Restricted to the Ileum, Cecum, and Colon of Vitamin D Receptor Knockout Mice Rescues Vitamin D Receptor-Dependent Rickets.Endocrinology. 2017 Nov 1;158(11):3792-3804. doi: 10.1210/en.2017-00258. Endocrinology. 2017. PMID: 28938396 Free PMC article.
-
In vivo function of VDR in gene expression-VDR knock-out mice.J Steroid Biochem Mol Biol. 1999 Apr-Jun;69(1-6):247-51. doi: 10.1016/s0960-0760(99)00042-4. J Steroid Biochem Mol Biol. 1999. PMID: 10418998 Review.
Cited by
-
Vitamin D Receptor (VDR) Genetic Variants: Relationship of FokI Genotypes with VDR Expression and Clinical Disease Activity in Systemic Lupus Erythematosus Patients.Genes (Basel). 2022 Nov 3;13(11):2016. doi: 10.3390/genes13112016. Genes (Basel). 2022. PMID: 36360253 Free PMC article.
-
Calcifediol During Pregnancy Improves Maternal and Fetal Availability of Vitamin D Compared to Vitamin D3 in Rats and Modifies Fetal Metabolism.Front Nutr. 2022 Apr 12;9:871632. doi: 10.3389/fnut.2022.871632. eCollection 2022. Front Nutr. 2022. PMID: 35495908 Free PMC article.
-
Expression of vitamin D receptor, CYP24A1, and CYP27B1 in normal and inflamed canine pancreases.Front Vet Sci. 2023 Sep 21;10:1265203. doi: 10.3389/fvets.2023.1265203. eCollection 2023. Front Vet Sci. 2023. PMID: 37808100 Free PMC article.
-
Vitamin D regulation of HAS2, hyaluronan synthesis and metabolism in triple negative breast cancer cells.J Steroid Biochem Mol Biol. 2020 Jul;201:105688. doi: 10.1016/j.jsbmb.2020.105688. Epub 2020 Apr 30. J Steroid Biochem Mol Biol. 2020. PMID: 32360595 Free PMC article.
-
Expression of Vitamin D Receptor and Vitamin D Receptor Gene Polymorphisms (BsmI, FokI, and TaqI) in Patients with Pterygium.Arq Bras Oftalmol. 2021 May-Jun;84(3):241-248. doi: 10.5935/0004-2749.20210032. Arq Bras Oftalmol. 2021. PMID: 33567021 Free PMC article.
References
-
- Pike JW, Meyer MB, Bishop KA. Regulation of target gene expression by the vitamin D receptor–an update on mechanisms. Rev Endocr Metab Disord. 2012;13(1):45–55. - PubMed
-
- Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78(4):1193–1231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

