Time Course of Evolution of Disability and Cause-Specific Mortality After Ischemic Stroke: Implications for Trial Design
- PMID: 28603141
- PMCID: PMC5669183
- DOI: 10.1161/JAHA.117.005788
Time Course of Evolution of Disability and Cause-Specific Mortality After Ischemic Stroke: Implications for Trial Design
Abstract
Background: Outcome in stroke trials is often based on a 3-month modified Rankin scale (mRS). How 3-month mRS relates to longer-term outcomes will depend on late recovery, delayed stroke-related deaths, recurrent strokes, and nonstroke deaths. We evaluated 3-month mRS and death/disability at 1 and 5 years in a population-based cohort study.
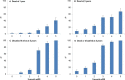
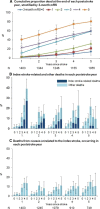
Methods and results: In 3-month survivors of ischemic stroke (Oxford Vascular Study; 2002-2014), we related 3-month mRS to disability (defined as mRS >2) at 1 and 5 years and/or death rates (age/sex adjusted). Accrual of disability and index-stroke-related and nonstroke deaths in each poststroke year was categorized according to 3-month mRS. Among 1606 patients with acute ischemic stroke, 181 died within 3 months, but 126 index-stroke-related deaths and 320 other deaths occurred during the subsequent 4866 patient-years of follow-up up to 5 years. Although 69/126 (54.8%) post-3-month index-stroke-related deaths occurred after 1 year, mRS>2 at 1 year strongly predicted these deaths (adjusted hazard ratio=21.94, 95%CI 7.88-61.09, P<0.0001). Consequently, a 3-month mRS >2 was a strong independent predictor of death at both 1 year (adjusted hazard ratio=6.67, 95%CI 4.16-10.69, P<0.0001) and 5 years (adjusted hazard ratio=2.93, 95%CI 2.38-3.60, P<0.0001). Although mRS improved by ≥1 point from 3 months to 1 year in 317/1266 (25.0%) patients with 3-month mRS ≥1, improvement in mRS after 1 year was limited (improvement by ≥1 point: 91/858 [10.6%]; improvement to mRS ≤2: 13/353 [3.7%]).
Conclusions: Our results reaffirm use of the 3-month mRS outcome in stroke trials. Although later recovery does occur, extending follow-up to 1 year would capture most long-term stroke-related disability. However, administrative mortality follow-up beyond 1 year has the potential to demonstrate translation of early disability gains into additional reductions in long-term mortality without much erosion by non-stroke-related deaths.
Keywords: cerebrovascular disease/stroke; clinical trial design; health economics; longitudinal cohort study; stroke recovery.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures


References
-
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W; Stroke Thrombolysis Trialists' Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. - PMC - PubMed
-
- Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, Broderick JP, Chen X, Chen G, Sharma VK, Kim JS, Thang NH, Cao Y, Parsons MW, Levi C, Huang Y, Olavarria VV, Demchuk AM, Bath PM, Donnan GA, Martins S, Pontes‐Neto OM, Silva F, Ricci S, Roffe C, Pandian J, Billot L, Woodward M, Li Q, Wang X, Wang J, Chalmers J; ENCHANTED Investigators and Coordinators . Low‐dose versus standard‐dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016;374:2313–2323. - PubMed
-
- Berge E, Cohen G, Roaldsen MB, Lundstrom E, Isaksson E, Rudberg AS, Slot KB, Forbes J, Smith J, Drever J, Wardlaw JM, Lindley RI, Sandercock PA, Whiteley WN; IST‐3 Collaborative Group . Effects of alteplase on survival after ischaemic stroke (IST‐3): 3 year follow‐up of a randomised, controlled, open‐label trial. Lancet Neurol. 2016;15:1028–1034. - PubMed
-
- Stroke Therapy Academic Industry Roundtable, II . Recommendations for clinical trial evaluation of acute stroke therapies. Stroke. 2001;32:1598–1606. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

