In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy
- PMID: 28603412
- PMCID: PMC5457178
- DOI: 10.2147/COPD.S115886
In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy
Abstract
Background: Combining in vitro mouth-throat deposition measurements, cascade impactor data and computational fluid dynamics (CFD) simulations, four different inhalers were compared which are indicated for chronic obstructive pulmonary disease (COPD) treatment.
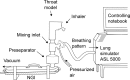
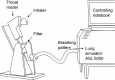
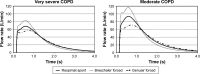
Methods: The Respimat inhaler, the Breezhaler, the Genuair, and the Ellipta were coupled to the idealized Alberta throat model. The modeled dose to the lung (mDTL) was collected downstream of the Alberta throat model using either a filter or a next generation impactor (NGI). Idealized breathing patterns from COPD patient groups - moderate and very severe COPD - were applied. Theoretical lung deposition patterns were assessed by an individual path model.
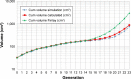
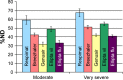
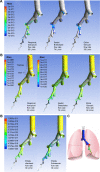
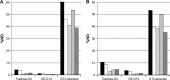
Results and conclusion: For the Respimat the mDTL was found to be 59% (SD 5%) for the moderate COPD breathing pattern and 67% (SD 5%) for very severe COPD breathing pattern. The percentages refer to nominal dose (ND) in vitro. This is in the range of 44%-63% in vivo in COPD patients who display large individual variability. Breezhaler showed a mDTL of 43% (SD 2%) for moderate disease simulation and 51% (SD 2%) for very severe simulation. The corresponding results for Genuair are mDTL of 32% (SD 2%) for moderate and 42% (SD 1%) for very severe disease. Ellipta vilanterol particles showed a mDTL of 49% (SD 3%) for moderate and 55% (SD 2%) for very severe disease simulation, and Ellipta fluticasone particles showed a mDTL of 33% (SD 3%) and 41% (SD 2%), respectively for the two breathing patterns. Based on the throat output and average flows of the different inhalers, CFD simulations were performed. Laminar and turbulent steady flow calculations indicated that deposition occurs mainly in the small airways. In summary, Respimat showed the lowest amount of particles depositing in the mouth-throat model and the highest amount reaching all regions of the simulation lung model.
Keywords: CFD; NGI; Respimat; inhalation; lung deposition; throat model.
Conflict of interest statement
Disclosure AC conducts a thesis sponsored by Boehringer Ingelheim (BI), the manufacturer of the Respimat inhaler. HW is an employee of BI. PL is consultant to BI. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
In Vitro Dosing Performance of the ELLIPTA® Dry Powder Inhaler Using Asthma and COPD Patient Inhalation Profiles Replicated with the Electronic Lung (eLung™).J Aerosol Med Pulm Drug Deliv. 2015 Dec;28(6):498-506. doi: 10.1089/jamp.2015.1225. Epub 2015 Sep 15. J Aerosol Med Pulm Drug Deliv. 2015. PMID: 26372465 Free PMC article.
-
In Silico Lung Deposition Profiles of Three Single-Inhaler Triple Therapies in Patients with COPD Using Functional Respiratory Imaging.Int J Chron Obstruct Pulmon Dis. 2025 Jun 27;20:2103-2116. doi: 10.2147/COPD.S510214. eCollection 2025. Int J Chron Obstruct Pulmon Dis. 2025. PMID: 40606813 Free PMC article.
-
Trelegy Ellipta--a three-drug inhaler for COPD.Med Lett Drugs Ther. 2018 May 21;60(1547):86-88. Med Lett Drugs Ther. 2018. PMID: 29913467 No abstract available.
-
Fluticasone furoate and vilanterol inhalation powder for the treatment of chronic obstructive pulmonary disease.Expert Rev Respir Med. 2015 Feb;9(1):5-12. doi: 10.1586/17476348.2015.986468. Epub 2014 Dec 6. Expert Rev Respir Med. 2015. PMID: 25482512 Review.
-
Relvar Ellipta for asthma.Drug Ther Bull. 2014 Aug;52(8):93-6. doi: 10.1136/dtb.2014.8.0273. Drug Ther Bull. 2014. PMID: 25125553 Review.
Cited by
-
Simulation of Airway Deposition of an Aerosol Drug in COPD Patients.Pharmaceutics. 2019 Apr 1;11(4):153. doi: 10.3390/pharmaceutics11040153. Pharmaceutics. 2019. PMID: 30939795 Free PMC article.
-
ENaC inhibition in cystic fibrosis: potential role in the new era of CFTR modulator therapies.Eur Respir J. 2020 Dec 24;56(6):2000946. doi: 10.1183/13993003.00946-2020. Print 2020 Dec. Eur Respir J. 2020. PMID: 32732328 Free PMC article. Review.
-
What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape.Ther Adv Respir Dis. 2019 Jan-Dec;13:1753466619884532. doi: 10.1177/1753466619884532. Ther Adv Respir Dis. 2019. PMID: 31805823 Free PMC article. Review.
-
Continued Innovation in Respiratory Care: The Importance of Inhaler Devices.Tuberc Respir Dis (Seoul). 2018 Apr;81(2):91-98. doi: 10.4046/trd.2017.0119. Tuberc Respir Dis (Seoul). 2018. PMID: 29589381 Free PMC article. Review.
-
TRONARTO: A Randomized, Placebo-Controlled Study of Tiotropium/Olodaterol Delivered via Soft Mist Inhaler in COPD Patients Stratified by Peak Inspiratory Flow.Int J Chron Obstruct Pulmon Dis. 2021 Aug 28;16:2455-2465. doi: 10.2147/COPD.S324467. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34511891 Free PMC article. Clinical Trial.
References
-
- Finlay WH. The mechanics of inhaled pharmaceutical aerosols. San Diego, CA: Academic Press Inc; 2001.
-
- Johnstone A, Uddin M, Pollard A, Heenan A, Finlay WH. The flow inside an idealised form of the human extra-thoracic airway. Experiments Fluids. 2004;37(5):673–689.
-
- Olsson B, Borgstrom L, Lundback H, Svensson M. Validation of a general in vitro approach for prediction of total lung deposition in healthy adults for pharmaceutical inhalation products. J Aerosol Med Pulm Drug Deliv. 2013;26(6):355–369. - PubMed
-
- Wachtel H, Bickmann D, Breitkreutz J, Langguth P. Can pediatric throat models and air flow profiles improve our dose finding strategy?; Paper presented at: Respiratory Drug Delivery; April 25–29, 2010; Orlando, Florida, USA.
-
- Wachtel H, Flüge T, Gössl R. Flow – pressure – energy – power: which is the essential factor in breathing patterns of patients using inhalers?; Paper presented at: Respiratory Drug Delivery; April 23–27, 2006; Boca Raton, Florida, USA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

