Sigma-1 Receptor Plays a Negative Modulation on N-type Calcium Channel
- PMID: 28603497
- PMCID: PMC5445107
- DOI: 10.3389/fphar.2017.00302
Sigma-1 Receptor Plays a Negative Modulation on N-type Calcium Channel
Abstract
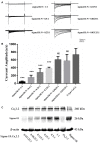
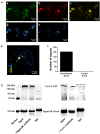
The sigma-1 receptor is a 223 amino acids molecular chaperone with a single transmembrane domain. It is resident to eukaryotic mitochondrial-associated endoplasmic reticulum and plasma membranes. By chaperone-mediated interactions with ion channels, G-protein coupled receptors and cell-signaling molecules, the sigma-1 receptor performs broad physiological and pharmacological functions. Despite sigma-1 receptors have been confirmed to regulate various types of ion channels, the relationship between the sigma-1 receptor and N-type Ca2+ channel is still unclear. Considering both sigma-1 receptors and N-type Ca2+ channels are involved in intracellular calcium homeostasis and neurotransmission, we undertake studies to explore the possible interaction between these two proteins. In the experiment, we confirmed the expression of the sigma-1 receptors and the N-type calcium channels in the cholinergic interneurons (ChIs) in rat striatum by using single-cell reverse transcription-polymerase chain reaction (scRT-PCR) and immunofluorescence staining. N-type Ca2+ currents recorded from ChIs in the brain slice of rat striatum was depressed when sigma-1 receptor agonists (SKF-10047 and Pre-084) were administrated. The inhibition was completely abolished by sigma-1 receptor antagonist (BD-1063). Co-expression of the sigma-1 receptors and the N-type calcium channels in Xenopus oocytes presented a decrease of N-type Ca2+ current amplitude with an increase of sigma-1 receptor expression. SKF-10047 could further depress N-type Ca2+ currents recorded from oocytes. The fluorescence resonance energy transfer (FRET) assays and co-immunoprecipitation (Co-IP) demonstrated that sigma-1 receptors and N-type Ca2+ channels formed a protein complex when they were co-expressed in HEK-293T (Human Embryonic Kidney -293T) cells. Our results revealed that the sigma-1 receptors played a negative modulation on N-type Ca2+ channels. The mechanism for the inhibition of sigma-1 receptors on N-type Ca2+ channels probably involved a chaperone-mediated direct interaction and agonist-induced conformational changes in the receptor-channel complexes on the cell surface.
Keywords: N-type Ca2+ channel; electrophysiology; ion channels modulation; protein–protein interaction; sigma-1 receptor.
Figures





Similar articles
-
Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons.J Neurophysiol. 2002 Jun;87(6):2867-79. doi: 10.1152/jn.2002.87.6.2867. J Neurophysiol. 2002. PMID: 12037190
-
Calcium currents in striatal fast-spiking interneurons: dopaminergic modulation of CaV1 channels.BMC Neurosci. 2018 Jul 16;19(1):42. doi: 10.1186/s12868-018-0441-0. BMC Neurosci. 2018. PMID: 30012109 Free PMC article.
-
The antidepressant-like effect induced by the sigma(1) (sigma(1)) receptor agonist igmesine involves modulation of intracellular calcium mobilization.Psychopharmacology (Berl). 2002 Aug;163(1):26-35. doi: 10.1007/s00213-002-1150-y. Epub 2002 Jun 29. Psychopharmacology (Berl). 2002. PMID: 12185397
-
The Roles of Intracellular Chaperone Proteins, Sigma Receptors, in Parkinson's Disease (PD) and Major Depressive Disorder (MDD).Front Pharmacol. 2019 May 21;10:528. doi: 10.3389/fphar.2019.00528. eCollection 2019. Front Pharmacol. 2019. PMID: 31178723 Free PMC article. Review.
-
Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.Dan Med Bull. 2010 Oct;57(10):B4191. Dan Med Bull. 2010. PMID: 21040688 Review.
Cited by
-
Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases.Front Neurosci. 2019 Aug 28;13:862. doi: 10.3389/fnins.2019.00862. eCollection 2019. Front Neurosci. 2019. PMID: 31551669 Free PMC article. Review.
-
Sigma-1 Receptor Modulates CFA-Induced Inflammatory Pain via Sodium Channels in Small DRG Neurons.Biomolecules. 2025 Jan 6;15(1):73. doi: 10.3390/biom15010073. Biomolecules. 2025. PMID: 39858467 Free PMC article.
-
Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values.Front Mol Biosci. 2019 Oct 18;6:91. doi: 10.3389/fmolb.2019.00091. eCollection 2019. Front Mol Biosci. 2019. PMID: 31750312 Free PMC article. Review.
-
PRE-084 as a tool to uncover potential therapeutic applications for selective sigma-1 receptor activation.Int J Biochem Cell Biol. 2020 Sep;126:105803. doi: 10.1016/j.biocel.2020.105803. Epub 2020 Jul 12. Int J Biochem Cell Biol. 2020. PMID: 32668330 Free PMC article. Review.
-
Sigma-1 Receptor: A Potential Therapeutic Target for Traumatic Brain Injury.Front Cell Neurosci. 2021 Sep 30;15:685201. doi: 10.3389/fncel.2021.685201. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34658788 Free PMC article. Review.
References
-
- Antonini V., Marrazzo A., Kleiner G., Coradazzi M., Ronsisvalle S., Prezzavento O., et al. (2011). Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (-)-MR22 in rats with selective cholinergic lesion and amyloid infusion. J. Alzheimers Dis. 24 569–586. 10.3233/JAD-2011-101794 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

