Synthesis and pharmacological evaluation of novel selective MOR agonist 6β-pyridinyl amidomorphines exhibiting long-lasting antinociception
- PMID: 28603600
- PMCID: PMC5464418
- DOI: 10.1039/C6MD00450D
Synthesis and pharmacological evaluation of novel selective MOR agonist 6β-pyridinyl amidomorphines exhibiting long-lasting antinociception
Abstract
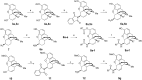
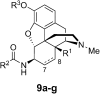
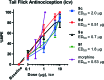
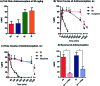
It was previously reported that 6β-aminomorphinan derivatives show high affinity for opiate receptors. Novel 6β-heteroarylamidomorphinanes were designed based on the MOR selective antagonist NAP. The 6β-aminomorphinanes were prepared with stereoselective Mitsunobu reaction and subsequently acylated with nicotinic acid and isonicotinic acid chloride hydrochlorides. The receptor binding and efficacy were determined in vitro and the analgesic activity was studied in vivo. The in vitro studies revealed moderate selectivity for MOR. At least two compounds in this series exhibited long-acting analgesic response when administered subcutaneously and intracerebroventricularly. When the substances were given intracerebroventricularly to mice, they showed analgesic potency comparable to morphine.
Conflict of interest statement
†The authors declare no competing interests.
Figures
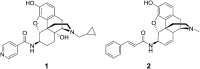
References
-
- Trescot A. M., Datta S., Lee M., Hansen H. Pain Physician. 2008;11:S133. - PubMed
-
- Chiara G. D., North R. A. Trends Pharmacol. Sci. 1992;13:185. - PubMed
-
- Loh H. H., Liu H.-C., Cavalli A., Yang W., Chen Y.-F., Wei L.-N. Mol. Brain Res. 1998;54:321. - PubMed
-
- Caruso T., Takemori A., Larson D., Portoghese P. Science. 1979;204:316. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

