The Type IX Secretion System (T9SS): Highlights and Recent Insights into Its Structure and Function
- PMID: 28603700
- PMCID: PMC5445135
- DOI: 10.3389/fcimb.2017.00215
The Type IX Secretion System (T9SS): Highlights and Recent Insights into Its Structure and Function
Abstract
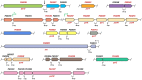
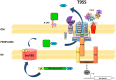
Protein secretion systems are vital for prokaryotic life, as they enable bacteria to acquire nutrients, communicate with other species, defend against biological and chemical agents, and facilitate disease through the delivery of virulence factors. In this review, we will focus on the recently discovered type IX secretion system (T9SS), a complex translocon found only in some species of the Bacteroidetes phylum. T9SS plays two roles, depending on the lifestyle of the bacteria. It provides either a means of movement (called gliding motility) for peace-loving environmental bacteria or a weapon for pathogens. The best-studied members of these two groups are Flavobacterium johnsoniae, a commensal microorganism often found in water and soil, and Porphyromonas gingivalis, a human oral pathogen that is a major causative agent of periodontitis. In P. gingivalis and some other periodontopathogens, T9SS translocates proteins, especially virulence factors, across the outer membrane (OM). Proteins destined for secretion bear a conserved C-terminal domain (CTD) that directs the cargo to the OM translocon. At least 18 proteins are involved in this still enigmatic process, with some engaged in the post-translational modification of T9SS cargo proteins. Upon translocation across the OM, the CTD is removed by a protease with sortase-like activity and an anionic LPS is attached to the newly formed C-terminus. As a result, a cargo protein could be secreted into the extracellular milieu or covalently attached to the bacterial surface. T9SS is regulated by a two-component system; however, the precise environmental signal that triggers it has not been identified. Exploring unknown systems contributing to bacterial virulence is exciting, as it may eventually lead to new therapeutic strategies. During the past decade, the major components of T9SS were identified, as well as hints suggesting the possible mechanism of action. In addition, the list of characterized cargo proteins is constantly growing. The actual structure of the translocon, situated in the OM of bacteria, remains the least explored area; however, new technical approaches and increasing scientific attention have resulted in a growing body of data. Therefore, we present a compact up-to-date review of this topic.
Keywords: Porphyromonas gingivalis; T9SS; gliding motility; pathogenesis; proteins; secretion; virulence.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

