Dishevelled Paralogs in Vertebrate Development: Redundant or Distinct?
- PMID: 28603713
- PMCID: PMC5445114
- DOI: 10.3389/fcell.2017.00059
Dishevelled Paralogs in Vertebrate Development: Redundant or Distinct?
Abstract
Dishevelled (DVL) proteins are highly conserved in the animal kingdom and are important key players in β-Catenin-dependent and -independent Wnt signaling pathways. Vertebrate genomes typically comprise three DVL genes, DVL1, DVL2, and DVL3. Expression patterns and developmental functions of the three vertebrate DVL proteins however, are only partially redundant in any given species. Moreover, expression and function of DVL isoforms have diverged between different vertebrate species. All DVL proteins share basic functionality in Wnt signal transduction. Additional, paralog-specific interactions and functions combined with context-dependent availability of DVL isoforms may play a central role in defining Wnt signaling specificity and add selectivity toward distinct downstream pathways. In this review, we recapitulate briefly cellular functions of DVL paralogs, their role in vertebrate embryonic development and congenital disease.
Keywords: Dishevelled; Wnt signaling; autosomal dominant robinow syndrome; embryonic expression; vertebrate embryonic development.
Figures
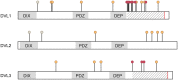
Similar articles
-
Roles of individual human Dishevelled paralogs in the Wnt signalling pathways.Cell Signal. 2021 Sep;85:110058. doi: 10.1016/j.cellsig.2021.110058. Epub 2021 May 31. Cell Signal. 2021. PMID: 34082011
-
Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development.PLoS Genet. 2008 Nov;4(11):e1000259. doi: 10.1371/journal.pgen.1000259. Epub 2008 Nov 14. PLoS Genet. 2008. PMID: 19008950 Free PMC article.
-
DVL mutations identified from human neural tube defects and Dandy-Walker malformation obstruct the Wnt signaling pathway.J Genet Genomics. 2020 Jun 20;47(6):301-310. doi: 10.1016/j.jgg.2020.06.003. Epub 2020 Jun 27. J Genet Genomics. 2020. PMID: 32900645
-
Decoding Dishevelled-Mediated Wnt Signaling in Vertebrate Early Development.Front Cell Dev Biol. 2020 Sep 30;8:588370. doi: 10.3389/fcell.2020.588370. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33102490 Free PMC article. Review.
-
Dishevelled: A masterful conductor of complex Wnt signals.Cell Signal. 2018 Jul;47:52-64. doi: 10.1016/j.cellsig.2018.03.004. Epub 2018 Mar 17. Cell Signal. 2018. PMID: 29559363 Free PMC article. Review.
Cited by
-
Nuclear Dishevelled targets gene regulatory regions and promotes tumor growth.EMBO Rep. 2021 Jun 4;22(6):e50600. doi: 10.15252/embr.202050600. Epub 2021 Apr 16. EMBO Rep. 2021. PMID: 33860601 Free PMC article.
-
Dishevelled-3 conformation dynamics analyzed by FRET-based biosensors reveals a key role of casein kinase 1.Nat Commun. 2019 Apr 18;10(1):1804. doi: 10.1038/s41467-019-09651-7. Nat Commun. 2019. PMID: 31000703 Free PMC article.
-
Exploring DIX-DIX Homo- and Hetero-Oligomers in Wnt Signaling with AlphaFold2.Cells. 2024 Oct 3;13(19):1646. doi: 10.3390/cells13191646. Cells. 2024. PMID: 39404409 Free PMC article.
-
Acetylation of conserved DVL-1 lysines regulates its nuclear translocation and binding to gene promoters in triple-negative breast cancer.Sci Rep. 2019 Nov 7;9(1):16257. doi: 10.1038/s41598-019-52723-3. Sci Rep. 2019. PMID: 31700102 Free PMC article.
-
Superresolution microscopy localizes endogenous Dvl2 to Wnt signaling-responsive biomolecular condensates.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2122476119. doi: 10.1073/pnas.2122476119. Epub 2022 Jul 22. Proc Natl Acad Sci U S A. 2022. PMID: 35867833 Free PMC article.
References
-
- Bossuyt J., Helmstadter K., Wu X., Clements-Jewery H., Haworth R. S., Avkiran M., et al. . (2008). Ca2+/calmodulin-dependent protein kinase IIdelta and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ. Res. 102, 695–702. 10.1161/CIRCRESAHA.107.169755 - DOI - PubMed
-
- Boutros M., Paricio N., Strutt D. I., Mlodzik M. (1998). Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94, 109–118. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

