Pretransplant 4β-hydroxycholesterol does not predict tacrolimus exposure or dose requirements during the first days after kidney transplantation
- PMID: 28603840
- PMCID: PMC5651327
- DOI: 10.1111/bcp.13343
Pretransplant 4β-hydroxycholesterol does not predict tacrolimus exposure or dose requirements during the first days after kidney transplantation
Abstract
Aims: The CYP3A metric 4β-hydroxycholesterol (4βOHC) has been shown to correlate with tacrolimus steady-state apparent oral clearance (CL/F). Recently, pretransplant 4βOHC was shown not to predict tacrolimus CL/F after transplantation in a cohort of renal recipients (n = 79). The goal of the current study was determine whether these findings could be validated in a substantially larger cohort.
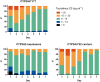
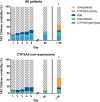
Methods: In a retrospective analysis of 279 renal recipients, tacrolimus trough concentrations (C0), daily dose, haematocrit and other relevant covariates were registered every day for the first 14 days after transplantation. 4βOHC and cholesterol were quantified on plasma collected immediately pretransplant using liquid chromatography tandem-mass spectrometry. Patients were genotyped for CYP3A5*1 and CYP3A4*22.
Results: A total of 3551 tacrolimus C0 concentrations were registered. In a linear mixed model for the 14-day period, determinants of tacrolimus C0 were CYP3A5 genotype, haematocrit, age and weight (overall R2 = 0.179). Determinants of daily dose were CYP3A5 genotype, age, methylprednisolone dose, tacrolimus formulation, ALT and estimated glomerular filtration rate (overall R2 = 0.242). Considering each of the first 5 days separately, 4βOHC had a limited effect on tacrolimus C0 on day 3 only (-1.00 ng ml-1 per ln, P = 0.035) but not on any other day, and no effect on dose or C0/dose. During the first 5 days, haematocrit and age, which were previously established as determinants of tacrolimus disposition under steady-state conditions, never explained more than 17.7% of between-subject variability in tacrolimus C0/dose.
Conclusions: The CYP3A metric 4βOHC cannot be used to predict tacrolimus dose requirements in the first days after transplantation.
Keywords: 4β-hydroxycholesterol; CYP3A4; CYP3A5; kidney transplantation; tacrolimus.
© 2017 The British Pharmacological Society.
Figures

Comment in
-
Was 4β-hydroxycholesterol ever going to be a useful marker of CYP3A4 activity?Br J Clin Pharmacol. 2018 Jul;84(7):1620-1621. doi: 10.1111/bcp.13538. Epub 2018 Feb 21. Br J Clin Pharmacol. 2018. PMID: 29464732 Free PMC article. No abstract available.
-
Kuypers and Vanhove reply to 'Was 4β-hydroxycholesterol ever going to be a useful marker of CYP3A4 activity?' by Neuhoff and Tucker.Br J Clin Pharmacol. 2018 Jul;84(7):1622-1623. doi: 10.1111/bcp.13592. Epub 2018 Apr 24. Br J Clin Pharmacol. 2018. PMID: 29691891 Free PMC article. No abstract available.
References
-
- Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: part II. Clin Pharmacokinet 2010; 49: 207–221. - PubMed
-
- de Jonge H, de Loor H, Verbeke K, Vanrenterghem Y, Kuypers DRJ. Impact of CYP3A5 genotype on tacrolimus versus midazolam clearance in renal transplant recipients: new insights in CYP3A5‐mediated drug metabolism. Pharmacogenomics 2013; 14: 1467–1480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

