Clinically Divergent Mutation Effects on the Structure and Function of the Human Cardiac Tropomyosin Overlap
- PMID: 28603979
- PMCID: PMC5575768
- DOI: 10.1021/acs.biochem.7b00266
Clinically Divergent Mutation Effects on the Structure and Function of the Human Cardiac Tropomyosin Overlap
Abstract
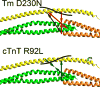
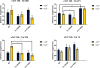
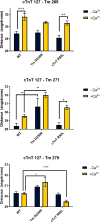
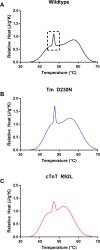
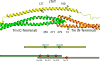
The progression of genetically inherited cardiomyopathies from an altered protein structure to clinical presentation of disease is not well understood. One of the main roadblocks to mechanistic insight remains a lack of high-resolution structural information about multiprotein complexes within the cardiac sarcomere. One example is the tropomyosin (Tm) overlap region of the thin filament that is crucial for the function of the cardiac sarcomere. To address this central question, we devised coupled experimental and computational modalities to characterize the baseline function and structure of the Tm overlap, as well as the effects of mutations causing divergent patterns of ventricular remodeling on both structure and function. Because the Tm overlap contributes to the cooperativity of myofilament activation, we hypothesized that mutations that enhance the interactions between overlap proteins result in more cooperativity, and conversely, those that weaken interaction between these elements lower cooperativity. Our results suggest that the Tm overlap region is affected differentially by dilated cardiomyopathy-associated Tm D230N and hypertrophic cardiomyopathy-associated human cardiac troponin T (cTnT) R92L. The Tm D230N mutation compacts the Tm overlap region, increasing the cooperativity of the Tm filament, contributing to a dilated cardiomyopathy phenotype. The cTnT R92L mutation causes weakened interactions closer to the N-terminal end of the overlap, resulting in decreased cooperativity. These studies demonstrate that mutations with differential phenotypes exert opposite effects on the Tm-Tn overlap, and that these effects can be directly correlated to a molecular level understanding of the structure and dynamics of the component proteins.
Figures



References
-
- Holmes KC, Lehman W. Gestalt-binding of tropomyosin to actin filaments. J. Muscle Res. Cell Motil. 2008;29:213–219. - PubMed
-
- Tardiff JC. Tropomyosin and dilated cardiomyopathy: revenge of the actinomyosin ″gatekeeper″. J. Am. Coll. Cardiol. 2010;55:330–332. - PubMed
-
- Bremel RD, Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat. New Biol. 1972;238:97–101. - PubMed
-
- Weber A, Murray JM. Molecular control mechanisms in muscle contraction. Physiol. Rev. 1973;53:612–673. - PubMed
-
- Tobacman LS, Sawyer D. Calcium binds cooperatively to the regulatory sites of the cardiac thin filament. J. Biol. Chem. 1990;265:931–939. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

