α-Conotoxin [S9A]TxID Potently Discriminates between α3β4 and α6/α3β4 Nicotinic Acetylcholine Receptors
- PMID: 28603989
- PMCID: PMC5572761
- DOI: 10.1021/acs.jmedchem.7b00546
α-Conotoxin [S9A]TxID Potently Discriminates between α3β4 and α6/α3β4 Nicotinic Acetylcholine Receptors
Abstract
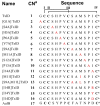
α3β4 nAChRs have been implicated in various pathophysiological conditions. However, the expression profile of α3β4 nAChRs and α6/α3β4 nAChRs overlap in a variety of tissues. To distinguish between these two subtypes, we redesigned peptide 1 (α-conotoxin TxID), which inhibits α3β4 and α6/α3β4 nAChR subtypes. We systematically mutated 1 to evaluate analogue selectivity for α3β4 vs α6/α3β4 nAChRs expressed in Xenopus laevis oocytes. One analogue, peptide 7 ([S9A]TxID), had 46-fold greater potency for α3β4 versus α6/α3β4 nAChRs. Peptide 7 had IC50s > 10 μM for other nAChR subtypes. Molecular dynamics simulations suggested that Ser-9 of TxID was involved in a weak hydrogen bond with β4 Lys-81 in the α6β4 binding site but not in the α3β4 binding site. When Ser-9 was substituted by an Ala, this hydrogen bond interaction was disrupted. These results provide further molecular insights into the selectivity of 7 and provide a guide for designing ligands that block α3β4 nAChRs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

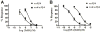

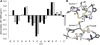

References
-
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. - PubMed
-
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. - PubMed
-
- Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137:22–54. - PubMed
-
- Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2015;96:302–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

