Efficacy of Mycobacterium vaccae immunotherapy for patients with tuberculosis: A systematic review and meta-analysis
- PMID: 28604170
- PMCID: PMC5612516
- DOI: 10.1080/21645515.2017.1335374
Efficacy of Mycobacterium vaccae immunotherapy for patients with tuberculosis: A systematic review and meta-analysis
Abstract
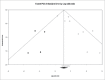
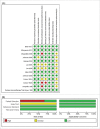
Tuberculosis (TB) is a significant cause of illness and death worldwide. Immunotherapy has been investigated in the treatment of TB. The purpose of this study was to perform a meta-analysis investigating the effectiveness of the M. vaccae vaccine. Medline, Cochrane, EMBASE, and Google Scholar were searched until November 5, 2015 using the keywords: tuberculosis, pulmonary TB, therapeutic vaccines, immunotherapy, M. vaccae, sputum smear. Randomized controlled trials (RCTs) or 2-arm prospective studies were included. The primary outcome was the sputum smear clearance rate at 1 or 2 months and 6 months after treatment. Secondary outcomes were improvement of chest X-ray findings, sputum culture negative rate at 1 or 2 months and 6 months, erythrocyte sedimentation rate (ESR), hemoglobin, and leukocyte count, weight gain, and mortality. Of 89 records identified, 13 RCTs were included in the meta-analysis. The number of patients ranged from 22 to 1337, and the mean age ranged from 26.4 to 44.3 y. Patients treated with M. vaccae were more likely to have negative sputum smear results at 1-2 months (pooled OR = 2.642, 95% CI: 1.623-4.301, P < .001) and at 6 months (pooled OR = 2.111, 95% CI: 1.141-3.908, P = .017), and have a negative sputum culture at 1 or 2 months (pooled OR = 2.660, 95% CI: 1.978-3.578, P < .001). The results of this meta-analysis suggest that M. vaccae immunotherapy may be effective in the treatment of pulmonary TB.
Keywords: Acid-fast bacilli; M. vaccae; immunotherapy; pulmonary; sputum smear; tuberculosis.
Figures
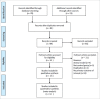
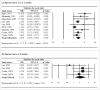
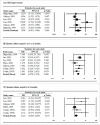
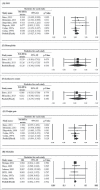



References
-
- World Health Organization (WHO) Global tuberculosis report 2015. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed: January20, 2016
-
- Montagnani C, Chiappini E, Galli L, de Martino M. Vaccine against tuberculosis: what's new? BMC Infect Dis 2014; 14 Suppl 1:S2; PMID:24564340; https://doi.org/ 10.1186/1471-2334-14-S1-S2 - DOI - PMC - PubMed
-
- Ahsan MJ. Recent advances in the development of vaccines for tuberculosis. Ther Adv Vaccines 2015; 3:66-75; PMID:26288734; https://doi.org/ 10.1177/2051013615593891 - DOI - PMC - PubMed
-
- Gröschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis–a systematic review. Vaccine 2014; 32:3162-8; PMID:24726245; https://doi.org/ 10.1016/j.vaccine.2014.03.047 - DOI - PubMed
-
- Bonickse R, Juhasz E. [Description of the new species Mycobacterium vaccae n. sp]. Zentralbl Bakteriol Orig 1964; 192:133-5. [Article in German] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
