Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies
- PMID: 28604384
- PMCID: PMC5490786
- DOI: 10.1172/JCI90597
Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies
Abstract
Solid organ transplantation is a curative therapy for hundreds of thousands of patients with end-stage organ failure. However, long-term outcomes have not improved, and nearly half of transplant recipients will lose their allografts by 10 years after transplant. One of the major challenges facing clinical transplantation is antibody-mediated rejection (AMR) caused by anti-donor HLA antibodies. AMR is highly associated with graft loss, but unfortunately there are few efficacious therapies to prevent and reverse AMR. This Review describes the clinical and histological manifestations of AMR, and discusses the immunopathological mechanisms contributing to antibody-mediated allograft injury as well as current and emerging therapies.
Conflict of interest statement
Figures
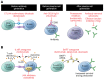
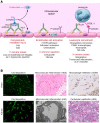

References
-
- Schinstock CA, et al. The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss. Am J Transplant. doi: 10.1111/ajt. [published online ahead of print December 15, 2016]. https://doi.org/10.1111/ajt.14161. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

