Impact of CYP2C19 Genotype and Liver Function on Voriconazole Pharmacokinetics in Renal Transplant Recipients
- PMID: 28604474
- PMCID: PMC5538305
- DOI: 10.1097/FTD.0000000000000425
Impact of CYP2C19 Genotype and Liver Function on Voriconazole Pharmacokinetics in Renal Transplant Recipients
Abstract
Background: Invasive fungal infection (IFI) is one of the leading causes of early death after renal transplantation. Voriconazole (VRC) is the first-line drug of IFI. Because of the large inter- and intraindividual variability in VRC plasma concentrations and the narrow therapeutic window for treating patients with IFIs, it is crucial to study the factors which could influence pharmacokinetic variability. We performed a population pharmacokinetics (PPK) study of VRC for personalized medicine.
Methods: A total of 125 trough concentrations (Cmin) from 56 patients were evaluated, retrospectively. Nonlinear mixed effect model was used to describe a PPK model that was internally validated by bootstrap method. Potential covariates included demographic characteristics, physiological and pathological data, concomitant medications, and CYP2C19 genotype.
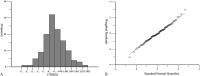
Results: A 1-compartment model with first-order absorption and elimination was fit to characterize the VRC pharmacokinetics in renal transplant recipients (RTRs). Aspartate aminotransferase (AST) had a significant influence on clearance (CL) while CYP2C19 genotype had a major impact on the volume of distribution (V). The parameters of CL and V were 4.76 L/h and 22.47 L, respectively. The final model was V (L) = 22.47 × [1 + 2.21 × (EM = 1)] × [1 + 4.67 × (IM = 1)] × [1 + 3.30 × (PM = 1)] × exp (0.96); CL (L/h) = 4.76 × (AST/33)^(-0.23) × exp (0.14). VRC Cmin in intermediate metabolizers was significantly higher than in extensive metabolizers.
Conclusions: Liver function and CYP2C19 polymorphism are major determinants of VRC pharmacokinetic variability in RTRs. Genotypes and clinical biomarkers can determine the initial scheme. Subsequently, therapeutic drug monitoring can optimize clinical efficacy and minimize toxicity. Hence, this is a feasible way to facilitate personalized medicine in RTRs. In addition, it is the first report about PPK of VRC in RTRs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. - PubMed
-
- López-Medrano F, Fernández-Ruiz M, Silva JT, et al. Clinical presentation and determinants of mortality of invasive pulmonary aspergillosis in kidney transplant recipients: a multinational cohort study. Am J Transpl. 2016;16:3220–3234. - PubMed
-
- López-Medrano F, Silva JT, Fernández-Ruiz M, et al. Risk factors associated with early invasive pulmonary aspergillosis in kidney transplant recipients: results from a multinational matched case-control study. Am J Transpl. 2016;16:2148–2157. - PubMed
-
- Heylen L, Maertens J, Naesens M, et al. Invasive aspergillosis after kidney transplant: case-control study. Clin Infect Dis. 2015;60:1505–1511. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

