Baccharis trimera (Less.) DC Exhibits an Anti-Adipogenic Effect by Inhibiting the Expression of Proteins Involved in Adipocyte Differentiation
- PMID: 28604636
- PMCID: PMC6152623
- DOI: 10.3390/molecules22060972
Baccharis trimera (Less.) DC Exhibits an Anti-Adipogenic Effect by Inhibiting the Expression of Proteins Involved in Adipocyte Differentiation
Abstract
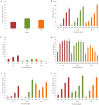
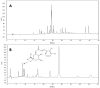
Baccharis trimera (Less.) DC (gorse) is a plant popularly used for the treatment of obesity. In this study, we prepared three B. trimera extracts aqueous extract (AE), decoction (AE-D), and methanol extract (ME) and investigated their antioxidant effects in six different tests and their anti-adipogenic effect in 3T3-L1 cells. The extracts showed a dose-dependent antioxidant activity in all tests. AE was the most potent antioxidant in copper and ferric ion chelation assays, whereas AE-D was the most potent in superoxide and hydroxyl radical scavenging assays, reducing power assay, and total antioxidant capacity analysis. Only ME showed a cytotoxic effect against 3T3-L1 cells. Lipid accumulation decreased in 3T3-L1 adipocytes in the presence of AE and AE-D extracts (0.5 to 1.0 mg/mL). In addition, the extracts dramatically attenuated the levels of adipogenic transcriptional factors, including CCAAT enhancer-binding protein α (C/EBPα), CCAAT enhancer-binding protein β (C/EBPβ), and gamma receptors by peroxisome proliferators (PPARγ), during adipogenesis. AE-D (1.0 mg/mL) caused an approximately 90% reduction in the levels of these molecules. We propose that B. trimera has an anti-adipogenic effect and could be used in the development of functional foods.
Keywords: adipogenesis; antioxidant; chlorogenic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- WHO Obesity and Overweight. [(accessed on 1 August 2016)]; Available online: http://www.who.int/mediacentre/factsheets/fs311/en/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

