Gibberellic Acid Signaling Is Required to Induce Flowering of Chrysanthemums Grown under Both Short and Long Days
- PMID: 28604637
- PMCID: PMC5486081
- DOI: 10.3390/ijms18061259
Gibberellic Acid Signaling Is Required to Induce Flowering of Chrysanthemums Grown under Both Short and Long Days
Abstract
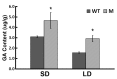
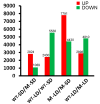
Flower bud formation and flowering in chrysanthemums occur under short day conditions (SD), but the molecular basis for the switch to reproductive growth is less well understood than in model plants. Here, a spontaneous mutant able to flower under long days is described. In an attempt to reveal the pathway(s) involved in the formation of flower buds under contrasting daylengths, transcriptome sequencing was carried out in plants grown both under SD and long day conditions (LD). A number of differentially transcribed genes involved in the various known flowering pathways were identified. Both circadian clock genes and Chrysanthemum FLOWERING LOCUS T Like3 (CmFTL3) were up-regulated under SD, thereby inducing floral bud formation and flowering. The gibberellin (GA) signaling pathway-related genes Gibberellin 20-oxidase (GA20ox) and Gibberellin receptor (GID1) were up-regulated in the mutant under LD, while the catabolic genes Gibberellin 2-oxidase (GA2ox) and GA-INSENSITIVE (GAI) were down-regulated, thereby inducing the transcription of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1) and LEAFY (LFY). The GA content of the leaf was higher in the mutant than in the wild type (WT) under LD and SD, and the mutant has more branching than WT plants under LD or SD. When treated with GA, the mutant flowered earlier under both SD and LD relative to WT, but there was no detectable phenotype difference between the two lines. The indication was that the photoperiod pathway majorly regulates flower bud formation and flowering time in chrysanthemums under SD. The GA signaling pathway only plays a subsidiary role for flowering. However, the GA signaling pathway predominated for flowering under LD.
Keywords: floral induction; flowering time; gibberellin; mutant; photoperiod.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Muller C.J.A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009;14:563–573. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

