Upregulation of mitochondrial NAD+ levels impairs the clonogenicity of SSEA1+ glioblastoma tumor-initiating cells
- PMID: 28604662
- PMCID: PMC5519015
- DOI: 10.1038/emm.2017.74
Upregulation of mitochondrial NAD+ levels impairs the clonogenicity of SSEA1+ glioblastoma tumor-initiating cells
Abstract
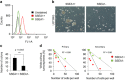
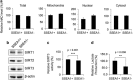
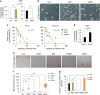
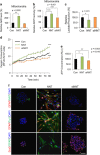
Emerging evidence has emphasized the importance of cancer therapies targeting an abnormal metabolic state of tumor-initiating cells (TICs) in which they retain stem cell-like phenotypes and nicotinamide adenine dinucleotide (NAD+) metabolism. However, the functional role of NAD+ metabolism in regulating the characteristics of TICs is not known. In this study, we provide evidence that the mitochondrial NAD+ levels affect the characteristics of glioma-driven SSEA1+ TICs, including clonogenic growth potential. An increase in the mitochondrial NAD+ levels by the overexpression of the mitochondrial enzyme nicotinamide nucleotide transhydrogenase (NNT) significantly suppressed the sphere-forming ability and induced differentiation of TICs, suggesting a loss of the characteristics of TICs. In addition, increased SIRT3 activity and reduced lactate production, which are mainly observed in healthy and young cells, appeared following NNT-overexpressed TICs. Moreover, in vivo tumorigenic potential was substantially abolished by NNT overexpression. Conversely, the short interfering RNA-mediated knockdown of NNT facilitated the maintenance of TIC characteristics, as evidenced by the increased numbers of large tumor spheres and in vivo tumorigenic potential. Our results demonstrated that targeting the maintenance of healthy mitochondria with increased mitochondrial NAD+ levels and SIRT3 activity could be a promising strategy for abolishing the development of TICs as a new therapeutic approach to treating aging-associated tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

