Oxidation of phosphorothioate DNA modifications leads to lethal genomic instability
- PMID: 28604692
- PMCID: PMC5577368
- DOI: 10.1038/nchembio.2407
Oxidation of phosphorothioate DNA modifications leads to lethal genomic instability
Abstract
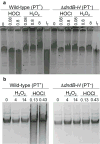
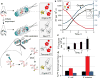
Genomic modification by sulfur in the form of phosphorothioate (PT) is widespread among prokaryotes, including human pathogens. Apart from its physiological functions, PT sulfur has redox and nucleophilic properties that suggest effects on bacterial fitness in stressful environments. Here we show that PTs are dynamic and labile DNA modifications that cause genomic instability during oxidative stress. In experiments involving isotopic labeling coupled with mass spectrometry, we observed sulfur replacement in PTs at a rate of ∼2% h-1 in unstressed Escherichia coli and Salmonella enterica. Whereas PT levels were unaffected by exposure to hydrogen peroxide (H2O2) or hypochlorous acid (HOCl), PT turnover increased to 3.8-10% h-1 after HOCl treatment and was unchanged by H2O2, consistent with the repair of HOCl-induced sulfur damage. PT-dependent sensitivity to HOCl extended to cytotoxicity and DNA strand breaks, which occurred at HOCl doses that were orders of magnitude lower than the corresponding doses of H2O2. The genotoxicity of HOCl in PT-containing bacteria suggests reduced fitness in competition with HOCl-producing organisms and during infections in humans.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Bayliss CD. Determinants of phase variation rate and the fitness implications of differing rates for bacterial pathogens and commensals. FEMS Microbiol Rev. 2009;33:504–520. - PubMed
-
- Sanchez-Romero MA, Cota I, Casadesus J. DNA methylation in bacteria: from the methyl group to the methylome. Curr Opin Microbiol. 2015;25:9–16. - PubMed
-
- Tock MR, Dryden DT. The biology of restriction and anti-restriction. Curr Opin Microbiol. 2005;8:466–472. - PubMed
-
- Wilson GG, Murray NE. Restriction and modification systems. Ann Rev Genetics. 1991;25:585–627. - PubMed
ONLINE METHODS REFERENCES
-
- Beers RF, Jr, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. - PubMed
-
- Held AM, Halko DJ, Hurst JK. Mechanisms of chlorine oxidation of hydrogen peroxide. J Am Chem Soc. 1978;100:5732–5740.
-
- Evans DF, Upton MW. Studies on singlet oxygen in aqueous solution. Part 1. Formation of singlet oxygen from hydrogen peroxide with two-electron oxidants. J Chem Soc Dalton Trans. 1985:1141–1145.
-
- Shams El Din AM, Mohammed RA. Kinetics of the reaction between hydrogen peroxide and hypochlorite. Desalination. 1998;115:145–153.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

