Fungal dysbiosis: immunity and interactions at mucosal barriers
- PMID: 28604735
- PMCID: PMC5724762
- DOI: 10.1038/nri.2017.55
Fungal dysbiosis: immunity and interactions at mucosal barriers
Abstract
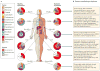
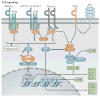
Fungi and mammals share a co-evolutionary history and are involved in a complex web of interactions. Studies focused on commensal bacteria suggest that pathological changes in the microbiota, historically known as dysbiosis, are at the root of many inflammatory diseases of non-infectious origin. However, the importance of dysbiosis in the fungal community - the mycobiota - was only recently acknowledged to have a pathological role, as novel findings have suggested that mycobiota disruption can have detrimental effects on host immunity. Fungal dysbiosis and homeostasis are dynamic processes that are probably more common than actual fungal infections, and therefore constantly shape the immune response. In this Review, we summarize specific mycobiota patterns that are associated with fungal dysbiosis, and discuss how mucosal immunity has evolved to distinguish fungal infections from dysbiosis and how it responds to these different conditions. We propose that gut microbiota dysbiosis is a collective feature of complex interactions between prokaryotic and eukaryotic microbial communities that can affect immunity and that can influence health and disease.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Peay KG, Kennedy PG, Talbot JM. Dimensions of biodiversity in the Earth mycobiome. Nature reviews Microbiology. 2016;14:434–447. - PubMed
-
- Wainright PO, Hinkle G, Sogin ML, Stickel SK. Monophyletic origins of the metazoa: an evolutionary link with fungi. Science. 1993;260:340–342. - PubMed
-
- Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–315. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

