Prostaglandin E2 inhibits Tr1 cell differentiation through suppression of c-Maf
- PMID: 28604806
- PMCID: PMC5467903
- DOI: 10.1371/journal.pone.0179184
Prostaglandin E2 inhibits Tr1 cell differentiation through suppression of c-Maf
Abstract
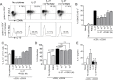
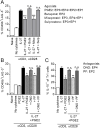
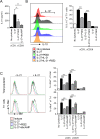
Prostaglandin E2 (PGE2), a major lipid mediator abundant at inflammatory sites, acts as a proinflammatory agent in models of inflammatory/autoimmune diseases by promoting CD4 Th1/Th17 differentiation. Regulatory T cells, including the IL-10 producing Tr1 cells counterbalance the proinflammatory activity of effector Th1/Th17 cells. Tr1 cell differentiation and function are induced by IL-27, and depend primarily on sustained expression of c-Maf in addition to AhR and Blimp-1. In agreement with the in vivo proinflammatory role of PGE2, here we report for the first time that PGE2 inhibits IL-27-induced differentiation and IL-10 production of murine CD4+CD49b+LAG-3+Foxp3- Tr1 cells. The inhibitory effect of PGE2 was mediated through EP4 receptors and induction of cAMP, leading to a significant reduction in c-Maf expression. Although PGE2 reduced IL-21 production in differentiating Tr1 cells, its inhibitory effect on Tr1 differentiation and c-Maf expression also occurred independent of IL-21 signaling. PGE2 did not affect STAT1/3 activation, AhR expression and only marginally reduced Egr-2/Blimp-1 expression. The effect of PGE2 on CD4+CD49b+LAG-3+ Tr1 differentiation was not associated with either induction of Foxp3 or IL-17 production, suggesting a lack of transdifferentiation into Foxp3+ Treg or effector Th17 cells. We recently reported that PGE2 inhibits the expression and production of IL-27 from activated conventional dendritic cells (cDC) in vivo and in vitro. The present study indicates that PGE2 also reduces murine Tr1 differentiation and function directly by acting on IL-27-differentiating Tr1 cells. Together, the ability of PGE2 to inhibit IL-27 production by cDC, and the direct inhibitory effect on Tr1 differentiation mediated through reduction in c-Maf expression, represent a new mechanistic perspective for the proinflammatory activity of PGE2.
Conflict of interest statement
Figures





References
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–64. - PubMed
-
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784 - DOI - PubMed
-
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko s, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389(6652):737–42. doi: 10.1038/39614 - DOI - PubMed
-
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19(6):739–46. doi: 10.1038/nm.3179 - DOI - PubMed
-
- Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, et al. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 2010;59(2):433–9. doi: 10.2337/db09-1168 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

