Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae
- PMID: 28604843
- PMCID: PMC5481032
- DOI: 10.1371/journal.ppat.1006426
Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae
Abstract
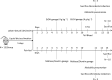
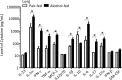
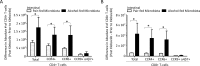
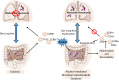
Chronic alcohol consumption perturbs the normal intestinal microbial communities (dysbiosis). To investigate the relationship between alcohol-mediated dysbiosis and pulmonary host defense we developed a fecal adoptive transfer model, which allows us to investigate the impact of alcohol-induced gut dysbiosis on host immune response to an infectious challenge at a distal organ, independent of prevailing alcohol use. Male C57BL/6 mice were treated with a cocktail of antibiotics (ampicillin, gentamicin, neomycin, vancomycin, and metronidazole) via daily gavage for two weeks. A separate group of animals was fed a chronic alcohol (or isocaloric dextrose pair-fed controls) liquid diet for 10 days. Microbiota-depleted mice were recolonized with intestinal microbiota from alcohol-fed or pair-fed (control) animals. Following recolonization groups of mice were sacrificed prior to and 48 hrs. post respiratory infection with Klebsiella pneumoniae. Klebsiella lung burden, lung immunology and inflammation, as well as intestinal immunology, inflammation, and barrier damage were examined. Results showed that alcohol-associated susceptibility to K. pneumoniae is, in part, mediated by gut dysbiosis, as alcohol-naïve animals recolonized with a microbiota isolated from alcohol-fed mice had an increased respiratory burden of K. pneumoniae compared to mice recolonized with a control microbiota. The increased susceptibility in alcohol-dysbiosis recolonized animals was associated with an increase in pulmonary inflammatory cytokines, and a decrease in the number of CD4+ and CD8+ T-cells in the lung following Klebsiella infection but an increase in T-cell counts in the intestinal tract following Klebsiella infection, suggesting intestinal T-cell sequestration as a factor in impaired lung host defense. Mice recolonized with an alcohol-dysbiotic microbiota also had increased intestinal damage as measured by increased levels of serum intestinal fatty acid binding protein. Collectively, these results suggest that alterations in the intestinal immune response as a consequence of alcohol-induced dysbiosis contribute to increased host susceptibility to Klebsiella pneumonia.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


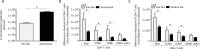

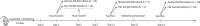


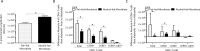


References
-
- Alcohol Facts and Statistics | National Institute on Alcohol Abuse and Alcoholism (NIAAA) 2016. Available from: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/al....
-
- Alcohol Use Disorder | National Institute on Alcohol Abuse and Alcoholism (NIAAA) 2016. Available from: http://www.ncbi.nlm.nih.gov/pubmed/.
-
- Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–32. Epub 2005/12/03. doi: 10.1513/pats.200507-065JS . - DOI - PubMed
-
- Jong GM, Hsiue TR, Chen CR, Chang HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107(1):214–7. Epub 1995/01/01. . - PubMed
-
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. Epub 2007/09/04. doi: 10.1016/j.alcohol.2007.06.003 ; PubMed Central PMCID: PMCPMC2081157. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

