Safety and Efficacy of Adding a Single Low Dose of Primaquine to the Treatment of Adult Patients With Plasmodium falciparum Malaria in Senegal, to Reduce Gametocyte Carriage: A Randomized Controlled Trial
- PMID: 28605472
- PMCID: PMC5848230
- DOI: 10.1093/cid/cix355
Safety and Efficacy of Adding a Single Low Dose of Primaquine to the Treatment of Adult Patients With Plasmodium falciparum Malaria in Senegal, to Reduce Gametocyte Carriage: A Randomized Controlled Trial
Abstract
Introduction: More information is needed about the safety of low-dose primaquine in populations where G6PD deficiency is common.
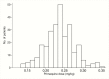
Methods: Adults with Plasmodium falciparum malaria were randomized to receive 1 of 3 artemisinin combination therapies (ACTs) with or without primaquine (0.25 mg/kg). Glucose-6-phosphate dehydrogenase (G6PD) status was determined using a rapid test. Patients were followed for 28 days to record hemoglobin concentration, adverse events, and gametocyte carriage. The primary end point was the change in Hb at day 7.
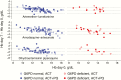
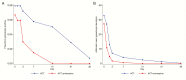
Results: In sum, 274 patients were randomized, 139 received an ACT alone, and 135 received an ACT + primaquine. The mean reduction in Hb at day 7 was similar in each group, a difference in the ACT + PQ versus the ACT alone group of -0.04 g/dL (95% confidence interval [CI] -0.23, 0.31), but the effect of primaquine differed according to G6PD status. In G6PD-deficient patients the drop in Hb was 0.63 g/dL (95% CI 0.03, 1.24) greater in those who received primaquine than in those who received an ACT alone. In G6PD-normal patients, the reduction in Hb was 0.22 g/dL (95% CI -0.08, 0.52) less in those who received primaquine (interaction P = .01). One G6PD normal patient who received primaquine developed moderately severe anaemia (Hb < 8 g/dL). Dark urine was more frequent in patients who received primaquine. Primaquine was associated with a 73% (95% CI 24-90) reduction in gametocyte carriage (P = .013).
Conclusion: Primaquine substantially reduced gametocyte carriage. However, the fall in Hb concentration at day 7 was greater in G6PD-deficient patients who received primaquine than in those who did not and one patient who received primaquine developed moderately severe anemia.
Clinical trial registration: PACTR201411000937373 (www.pactr.org).
Keywords: Senegal; hemoglobin; plasmodium; primaquine; safety.
© The Author 2017. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- WHO. World malaria report 2016. Geneva: World Health Organization, 2016.
-
- WHO. Updated WHO policy recommendation (October 2012): single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Geneva: WHO; Global Malaria Program, 2012.
-
- Recht J, Ashley E, White N.. Safety of 8-aminoquinoline antimalarial medicines. Geneva: World Health Organization, 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

