MerTK as a therapeutic target in glioblastoma
- PMID: 28605477
- PMCID: PMC5761530
- DOI: 10.1093/neuonc/nox111
MerTK as a therapeutic target in glioblastoma
Abstract
Background: Glioma-associated macrophages and microglia (GAMs) are components of the glioblastoma (GBM) microenvironment that express MerTK, a receptor tyrosine kinase that triggers efferocytosis and can suppress innate immune responses. The aim of the study was to define MerTK as a therapeutic target using an orally bioavailable inhibitor, UNC2025.
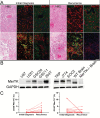
Methods: We examined MerTK expression in tumor cells and macrophages in matched patient GBM samples by double-label immunohistochemistry. UNC2025-induced MerTK inhibition was studied in vitro and in vivo.
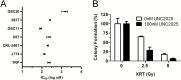
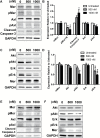
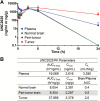
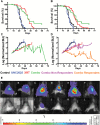
Results: MerTK/CD68+ macrophages increased in recurrent tumors while MerTK/glial fibrillary acidic protein-positive tumor cells did not. Pharmacokinetic studies showed high tumor exposures of UNC2025 in a syngeneic orthotopic allograft mouse GBM model. The same model mice were randomized to receive vehicle, daily UNC2025, fractionated external beam radiotherapy (XRT), or UNC2025/XRT. Although median survival (21, 22, 35, and 35 days, respectively) was equivalent with or without UNC2025, bioluminescence imaging (BLI) showed significant growth delay with XRT/UNC2025 treatment and complete responses in 19%. The responders remained alive for 60 days and showed regression to 1%-10% of pretreatment BLI tumor burden; 5 of 6 were tumor free by histology. In contrast, only 2% of 98 GBM mice of the same model treated with XRT survived 50 days and none survived 60 days. UNC2025 also reduced CD206+ macrophages in mouse tumor samples.
Conclusions: These results suggest that MerTK inhibition combined with XRT has a therapeutic effect in a subset of GBM. Further mechanistic studies are warranted.
Keywords: MerTK; glioblastoma; macrophage; microenvironment.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2017. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Robert C, Schachter J, Long GV et al. ; KEYNOTE-006 investigators. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

