Targeted protein knockdown using small molecule degraders
- PMID: 28605671
- PMCID: PMC5584562
- DOI: 10.1016/j.cbpa.2017.05.016
Targeted protein knockdown using small molecule degraders
Abstract
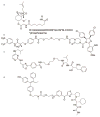
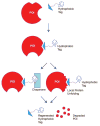
Small molecule probes of biological systems have traditionally been designed to bind to and inhibit the active sites of their protein targets. While this class of pharmacological agents has been broadened by the development of a small number of allosteric and protein-protein interaction (PPI) inhibitors, conventional drug design still excludes 'undruggable' proteins that are neither enzymes nor receptors. Recent years have seen the emergence of new classes of small molecules that can target hitherto undruggable proteins by recruiting the cellular proteostasis machinery to selectively tag them for degradation. These molecules, especially the class known as Proteolysis Targeting Chimera (PROTACs), represent a paradigm shift in chemical genetics, but their most tantalizing potential is as novel therapeutic agents. This review briefly summarizes the preclinical development of small molecule-based protein degraders, and describes the recent improvements in the technology that have positioned PROTACs on the cusp of entering the clinic.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures

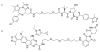
References
-
- Smietana K, Siatkowski M, Møller M. Trends in clinical success rates. Nat Rev Drug Discov. 2016;15:379–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

