Suppression of experimental cerebral malaria by disruption of malate:quinone oxidoreductase
- PMID: 28606087
- PMCID: PMC5469008
- DOI: 10.1186/s12936-017-1898-5
Suppression of experimental cerebral malaria by disruption of malate:quinone oxidoreductase
Abstract
Background: Aspartate, which is converted from oxaloacetate (OAA) by aspartate aminotransferase, is considered an important precursor for purine salvage and pyrimidine de novo biosynthesis, and is thus indispensable for the growth of Plasmodium parasites at the asexual blood stages. OAA can be produced in malaria parasites via two routes: (i) from phosphoenolpyruvate (PEP) by phosphoenolpyruvate carboxylase (PEPC) in the cytosol, or (ii) from fumarate by consecutive reactions catalyzed by fumarate hydratase (FH) and malate:quinone oxidoreductase (MQO) in the mitochondria of malaria parasites. Although PEPC-deficient Plasmodium falciparum and Plasmodium berghei (rodent malaria) parasites show a growth defect, the mutant P. berghei can still cause experimental cerebral malaria (ECM) with similar dynamics to wild-type parasites. In contrast, the importance of FH and MQO for parasite viability, growth and virulence is not fully understood because no FH- and MQO-deficient P. falciparum has been established. In this study, the role of FH and MQO in the pathogenicity of asexual-blood-stage Plasmodium parasites causing cerebral malaria was examined.
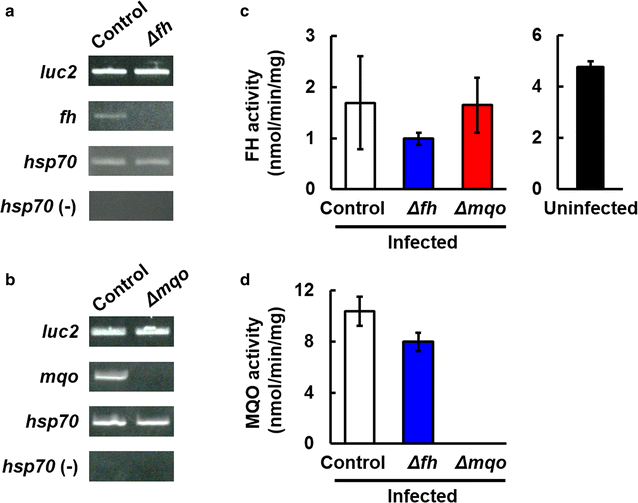
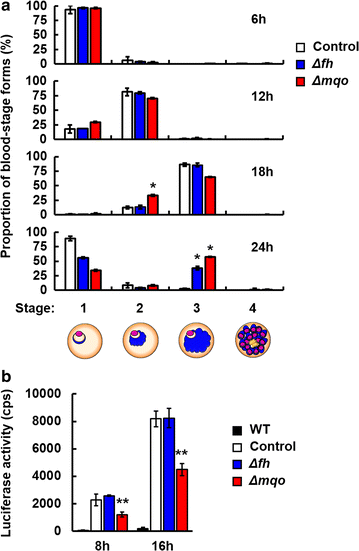
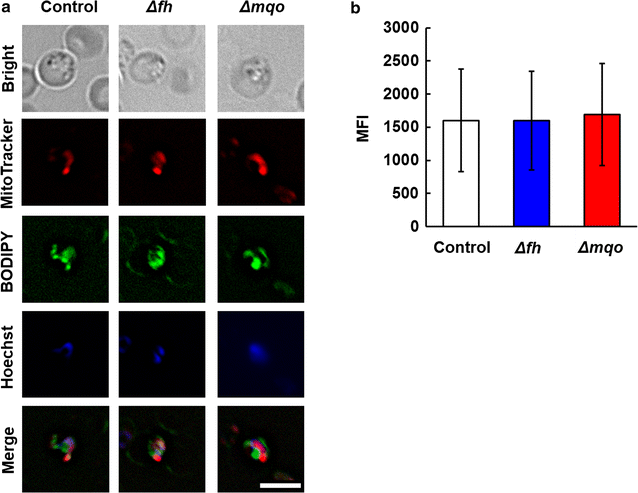
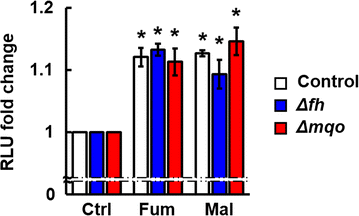
Results: First, FH- and MQO-deficient parasites were generated by inserting a luciferase-expressing cassette into the fh and mqo loci in the genome of P. berghei ANKA strain. Second, the viability of FH-deficient and MQO-deficient parasites that express luciferase was determined by measuring luciferase activity, and the effect of FH or MQO deficiency on the development of ECM was examined. While the viability of FH-deficient P. berghei was comparable to that of control parasites, MQO-deficient parasites exhibited considerably reduced viability. FH activity derived from erythrocytes was also detected. This result and the absence of phenotype in FH-deficient P. berghei parasites suggest that fumarate can be metabolized to malate by host or parasite FH in P. berghei-infected erythrocytes. Furthermore, although the growth of FH- and MQO-deficient parasites was impaired, the development of ECM was suppressed only in mice infected with MQO-deficient parasites.
Conclusions: These findings suggest that MQO-mediated mitochondrial functions are required for development of ECM of asexual-blood-stage Plasmodium parasites.
Keywords: Fumarate hydratase (FH); Luciferase–luciferin system; Malate:quinone oxidoreductase (MQO); Plasmodium berghei.
Figures

Similar articles
-
Identification of Plasmodium falciparum Mitochondrial Malate: Quinone Oxidoreductase Inhibitors from the Pathogen Box.Genes (Basel). 2019 Jun 21;10(6):471. doi: 10.3390/genes10060471. Genes (Basel). 2019. PMID: 31234346 Free PMC article.
-
Biochemical characterization and essentiality of Plasmodium fumarate hydratase.J Biol Chem. 2018 Apr 20;293(16):5878-5894. doi: 10.1074/jbc.M117.816298. Epub 2018 Feb 15. J Biol Chem. 2018. PMID: 29449371 Free PMC article.
-
Metabolic changes accompanying the loss of fumarate hydratase and malate-quinone oxidoreductase in the asexual blood stage of Plasmodium falciparum.J Biol Chem. 2022 May;298(5):101897. doi: 10.1016/j.jbc.2022.101897. Epub 2022 Apr 6. J Biol Chem. 2022. PMID: 35398098 Free PMC article.
-
Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei.Mamm Genome. 2018 Aug;29(7-8):488-506. doi: 10.1007/s00335-018-9752-9. Epub 2018 Jun 19. Mamm Genome. 2018. PMID: 29922917 Review.
-
An alternative approach to malaria vaccine with a permanent attenuated mutant from a high virulence Plasmodium berghei strain.Zentralbl Bakteriol Mikrobiol Hyg A. 1987 May;264(3-4):319-25. Zentralbl Bakteriol Mikrobiol Hyg A. 1987. PMID: 3310458 Review.
Cited by
-
Structural and Biochemical Features of Eimeria tenella Dihydroorotate Dehydrogenase, a Potential Drug Target.Genes (Basel). 2020 Dec 7;11(12):1468. doi: 10.3390/genes11121468. Genes (Basel). 2020. PMID: 33297567 Free PMC article.
-
Novel Characteristics of Mitochondrial Electron Transport Chain from Eimeria tenella.Genes (Basel). 2019 Jan 8;10(1):29. doi: 10.3390/genes10010029. Genes (Basel). 2019. PMID: 30626105 Free PMC article.
-
Identification of Plasmodium falciparum Mitochondrial Malate: Quinone Oxidoreductase Inhibitors from the Pathogen Box.Genes (Basel). 2019 Jun 21;10(6):471. doi: 10.3390/genes10060471. Genes (Basel). 2019. PMID: 31234346 Free PMC article.
-
The apicoplast localized isocitrate dehydrogenase is needed for de novo fatty acid synthesis in the apicoplast of Toxoplasma gondii.Front Cell Infect Microbiol. 2025 Jun 24;15:1542122. doi: 10.3389/fcimb.2025.1542122. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40630641 Free PMC article.
-
Biochemical characterization and essentiality of Plasmodium fumarate hydratase.J Biol Chem. 2018 Apr 20;293(16):5878-5894. doi: 10.1074/jbc.M117.816298. Epub 2018 Feb 15. J Biol Chem. 2018. PMID: 29449371 Free PMC article.
References
-
- WHO. World malaria report 2016. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report.... Accessed 4 Jan 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

