The importance of patient compliance in repeated rounds of mass drug administration (MDA) for the elimination of intestinal helminth transmission
- PMID: 28606164
- PMCID: PMC5469187
- DOI: 10.1186/s13071-017-2206-5
The importance of patient compliance in repeated rounds of mass drug administration (MDA) for the elimination of intestinal helminth transmission
Abstract
Background: Systematic non-compliance to chemotherapeutic treatment among a portion of the eligible population is thought to be a major obstacle to the elimination of helminth infections by mass drug administration (MDA). MDA for helminths is repeated at defined intervals such as yearly or every 2 years, as a consequence of the inability of the human host to develop fully protective immunity to reinfection. As such, how an individual complies to these repeated rounds of MDA can have a significant impact on parasite transmission. The importance of this factor is poorly understood at present. Few epidemiological studies have examined longitudinal trends in compliance in the many communities in areas of endemic helminth infection that are undergoing MDA. Reducing systematic non-compliance will obviously increase the number of individuals treated, but it may also alter the dynamics of parasite transmission.
Methods: Here we develop an individual-based stochastic model of helminth transmission and MDA treatment to investigate how different patterns of compliance influence the impact of MDA for two groups of helminths, the soil transmitted nematode infections and the schistosome parasites. We study the effect of several alternative treatment and compliance patterns on the dynamics of transmission.
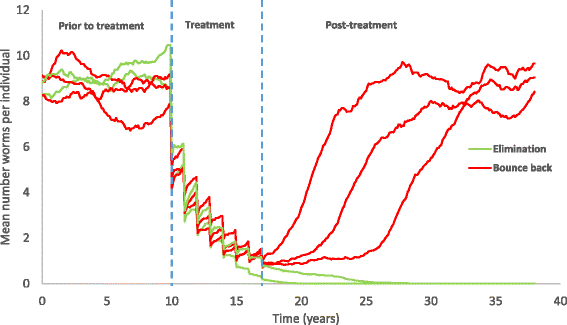
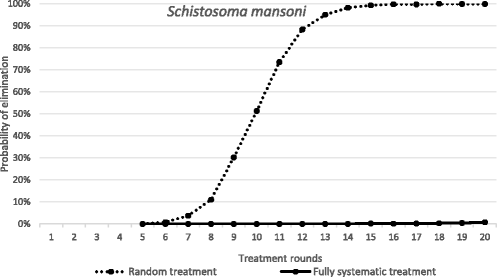
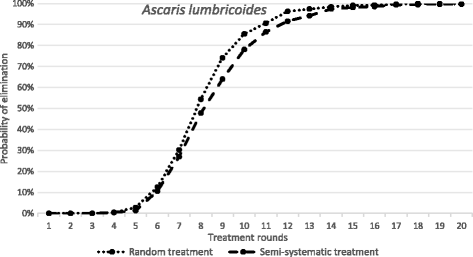
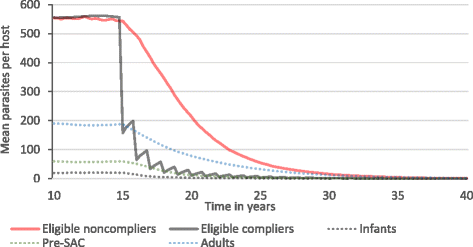
Results: We find that the impact of different compliance patterns, ranging from random treatment at each round of chemotherapy to systematic non-compliance by a proportion of the population, is very dependent on both transmission intensity in a defined setting and the type of infection that the treatment is targeted at. Systematic non-compliance has a greater impact on the potential for elimination of Schistosoma mansoni transmission by intensive MDA, than it does on Ascaris lumbricoides.
Conclusions: We discuss the implications of our findings for the prioritisation of resources in MDA programmes and for monitoring and evaluation programme design. The key message generated by the analyses is that great care must be taken to record individual longitudinal patterns of compliance at each round of MDA as opposed to just recording overall coverage.
Keywords: Compliance; Mass Drug Administration; Mathematical modelling; Schistosomiasis; Soil-transmitted Helminths; Systematic non-compliance.
Figures




References
-
- Anderson RM, Turner HC, Truscott JE, Hollingsworth TD, Brooker SJ. Should the goal for the treatment of soil transmitted helminth (STH) infections be changed from morbidity control in children to community-wide transmission elimination? PLoS Negl Trop Dis. 2015;9(8):e0003897. doi: 10.1371/journal.pntd.0003897. - DOI - PMC - PubMed
-
- Easton AV, Oliveira RG, O’Connell EM, Kepha S, Mwandawiro CS, Njenga SM, et al. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasit Vectors. 2016;9:38. doi: 10.1186/s13071-016-1314-y. - DOI - PMC - PubMed
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. doi: 10.1016/S0140-6736(12)61689-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

