Practical Measures of Clinical Benefit With Ruxolitinib Therapy: An Exploratory Analysis of COMFORT-I
- PMID: 28606598
- PMCID: PMC8148882
- DOI: 10.1016/j.clml.2017.05.015
Practical Measures of Clinical Benefit With Ruxolitinib Therapy: An Exploratory Analysis of COMFORT-I
Abstract
Background: The phase III COMFORT (Controlled Myelofibrosis Study With Oral JAK inhibitor Treatment)-I and COMFORT-II trials in patients with intermediate-2 or high-risk myelofibrosis (MF) showed that ruxolitinib was superior to placebo and best available therapy, respectively, for improvements in spleen volume, MF-related symptoms, and overall survival (OS). However, patients managed in community settings might not have access to the methods used in the COMFORT trials. In this exploratory analysis we summarize efficacy findings of COMFORT-I using practical, community-oriented measures of patient outcomes.
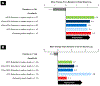
Patients and methods: In this post hoc analysis of data from COMFORT-I we evaluated changes from baseline to week 12 in spleen size (palpable length and volume), patient-reported outcomes (Patient Global Impression of Change; Myelofibrosis Symptom Assessment Form; Patient-Reported Outcomes Measurement System Fatigue Scale), body weight, and serum albumin levels in 5 subgroups of ruxolitinib-treated patients on the basis of week 12 spleen length changes from baseline: (1-4) ≥ 50%, 25% to < 50%, 10% to < 25%, or < 10% reduction; and (5) worsening. OS was evaluated in ruxolitinib-treated patients with week 12 spleen length reductions from baseline ≥ 50%, 25% to < 50%, or < 25% (including worsening).
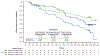
Results: In all spleen length subgroups, including patients with worsening spleen length at week 12, ruxolitinib (n = 150) was associated with improvements in spleen volume, patient-reported symptom burden, body weight, and serum albumin levels. Greater reductions in spleen length were associated with prolonged OS.
Conclusion: A variety of assessment methods beyond palpable spleen length that are easily accessible in the community setting might be useful in evaluating the clinical benefit of ruxolitinib over time in patients with MF.
Trial registration: ClinicalTrials.gov NCT00952289.
Keywords: Community hospitals; Janus kinases; Myelofibrosis; Myeloproliferative disorders; Splenomegaly.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2008; 22:14–22. - PubMed
-
- Gregory SA, Mesa RA, Hoffman R, Shammo JM. Clinical and laboratory features of myelofibrosis and limitations of current therapies. Clin Adv Hematol Oncol 2011; 9:1–16. - PubMed
-
- Mesa RA, Shields A, Hare T, et al. Progressive burden of myelofibrosis in untreated patients: assessment of patient-reported outcomes in patients randomized to placebo in the COMFORT-I study. Leuk Res 2013; 37:911–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

