Na/K pump inactivation, subsarcolemmal Na measurements, and cytoplasmic ion turnover kinetics contradict restricted Na spaces in murine cardiac myocytes
- PMID: 28606910
- PMCID: PMC5496509
- DOI: 10.1085/jgp.201711780
Na/K pump inactivation, subsarcolemmal Na measurements, and cytoplasmic ion turnover kinetics contradict restricted Na spaces in murine cardiac myocytes
Abstract
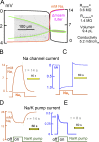
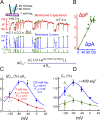
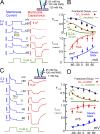
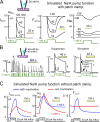
Decades ago, it was proposed that Na transport in cardiac myocytes is modulated by large changes in cytoplasmic Na concentration within restricted subsarcolemmal spaces. Here, we probe this hypothesis for Na/K pumps by generating constitutive transsarcolemmal Na flux with the Na channel opener veratridine in whole-cell patch-clamp recordings. Using 25 mM Na in the patch pipette, pump currents decay strongly during continuous activation by extracellular K (τ, ∼2 s). In contradiction to depletion hypotheses, the decay becomes stronger when pump currents are decreased by hyperpolarization. Na channel currents are nearly unchanged by pump activity in these conditions, and conversely, continuous Na currents up to 0.5 nA in magnitude have negligible effects on pump currents. These outcomes are even more pronounced using 50 mM Li as a cytoplasmic Na congener. Thus, the Na/K pump current decay reflects mostly an inactivation mechanism that immobilizes Na/K pump charge movements, not cytoplasmic Na depletion. When channel currents are increased beyond 1 nA, models with unrestricted subsarcolemmal diffusion accurately predict current decay (τ ∼15 s) and reversal potential shifts observed for Na, Li, and K currents through Na channels opened by veratridine, as well as for Na, K, Cs, Li, and Cl currents recorded in nystatin-permeabilized myocytes. Ion concentrations in the pipette tip (i.e., access conductance) track without appreciable delay the current changes caused by sarcolemmal ion flux. Importantly, cytoplasmic mixing volumes, calculated from current decay kinetics, increase and decrease as expected with osmolarity changes (τ >30 s). Na/K pump current run-down over 20 min reflects a failure of pumps to recover from inactivation. Simulations reveal that pump inactivation coupled with Na-activated recovery enhances the rapidity and effectivity of Na homeostasis in cardiac myocytes. In conclusion, an autoregulatory mechanism enhances cardiac Na/K pump activity when cytoplasmic Na rises and suppresses pump activity when cytoplasmic Na declines.
© 2017 Lu and Hilgemann.
Figures











Comment in
-
No fuzzy space for intracellular Na+ in healthy ventricular myocytes.J Gen Physiol. 2017 Jul 3;149(7):683-687. doi: 10.1085/jgp.201711826. Epub 2017 Jun 16. J Gen Physiol. 2017. PMID: 28626063 Free PMC article.
Similar articles
-
Profound regulation of Na/K pump activity by transient elevations of cytoplasmic calcium in murine cardiac myocytes.Elife. 2016 Sep 14;5:e19267. doi: 10.7554/eLife.19267. Elife. 2016. PMID: 27627745 Free PMC article.
-
Glutathionylation-Dependence of Na(+)-K(+)-Pump Currents Can Mimic Reduced Subsarcolemmal Na(+) Diffusion.Biophys J. 2016 Mar 8;110(5):1099-109. doi: 10.1016/j.bpj.2016.01.014. Biophys J. 2016. PMID: 26958887 Free PMC article.
-
Ion fluxes in giant excised cardiac membrane patches detected and quantified with ion-selective microelectrodes.J Gen Physiol. 2003 Apr;121(4):325-47. doi: 10.1085/jgp.200208777. J Gen Physiol. 2003. PMID: 12668735 Free PMC article.
-
Molecular control of cardiac sodium homeostasis in health and disease.J Cardiovasc Electrophysiol. 2006 May;17 Suppl 1:S47-S56. doi: 10.1111/j.1540-8167.2006.00383.x. J Cardiovasc Electrophysiol. 2006. PMID: 16686682 Review.
-
Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise.Physiol Rev. 2000 Oct;80(4):1411-81. doi: 10.1152/physrev.2000.80.4.1411. Physiol Rev. 2000. PMID: 11015618 Review.
Cited by
-
Hypertrophy of human embryonic stem cell-derived cardiomyocytes supported by positive feedback between Ca2+ and diacylglycerol signals.Pflugers Arch. 2019 Aug;471(8):1143-1157. doi: 10.1007/s00424-019-02293-0. Epub 2019 Jun 28. Pflugers Arch. 2019. PMID: 31250095 Free PMC article.
-
Regulation of Cardiac Contractility by the Alpha 2 Subunit of the Na+/K+-ATPase.Front Physiol. 2022 Jun 22;13:827334. doi: 10.3389/fphys.2022.827334. eCollection 2022. Front Physiol. 2022. PMID: 35812308 Free PMC article. Review.
-
ArrhythmoGenoPharmacoTherapy.Front Pharmacol. 2020 May 12;11:616. doi: 10.3389/fphar.2020.00616. eCollection 2020. Front Pharmacol. 2020. PMID: 32477118 Free PMC article. Review.
-
FXYD protein isoforms differentially modulate human Na/K pump function.J Gen Physiol. 2020 Dec 7;152(12):e202012660. doi: 10.1085/jgp.202012660. J Gen Physiol. 2020. PMID: 33231612 Free PMC article.
-
Regulation of ion transport from within ion transit pathways.J Gen Physiol. 2020 Jan 6;152(1):e201912455. doi: 10.1085/jgp.201912455. J Gen Physiol. 2020. PMID: 31875225 Free PMC article. Review.
References
-
- Akera T., Bennett R.T., Olgaard M.K., and Brody T.M.. 1976. Cardiac Na+, K+-adenosine triphosphatase inhibition by ouabain and myocardial sodium: A computer simulation. J. Pharmacol. Exp. Ther. 199:287–297. - PubMed
-
- Apell H.J., Schneeberger A., and Sokolov V.S.. 1998. Partial reactions of the Na,K-ATPase: Kinetic analysis and transport properties. Acta Physiol. Scand. Suppl. 643:235–245. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

