Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer
- PMID: 28606920
- PMCID: PMC6175044
- DOI: 10.1158/1078-0432.CCR-17-0369
Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer
Abstract
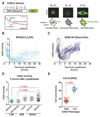
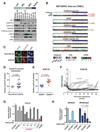
Purpose: Triple-negative breast cancer (TNBC) is a heterogeneous subgroup of breast cancer that is associated with a poor prognosis. We evaluated the activity of CDK4/6 inhibitors across the TNBC subtypes and investigated mechanisms of sensitivity.Experimental Design: A panel of cell lines representative of TNBC was tested for in vitro and in vivo sensitivity to CDK4/6 inhibition. A fluorescent CDK2 activity reporter was used for single-cell analysis in conjunction with time-lapse imaging.Results: The luminal androgen receptor (LAR) subtype of TNBC was highly sensitive to CDK4/6 inhibition both in vitro (P < 0.001 LAR vs. basal-like) and in vivo in MDA-MB-453 LAR cell line xenografts. Single-cell analysis of CDK2 activity demonstrated differences in cell-cycle dynamics between LAR and basal-like cells. Palbociclib-sensitive LAR cells exit mitosis with low levels of CDK2 activity, into a quiescent state that requires CDK4/6 activity for cell-cycle reentry. Palbociclib-resistant basal-like cells exit mitosis directly into a proliferative state, with high levels of CDK2 activity, bypassing the restriction point and the requirement for CDK4/6 activity. High CDK2 activity after mitosis is driven by temporal deregulation of cyclin E1 expression. CDK4/6 inhibitors were synergistic with PI3 kinase inhibitors in PIK3CA-mutant TNBC cell lines, extending CDK4/6 inhibitor sensitivity to additional TNBC subtypes.Conclusions: Cell-cycle dynamics determine the response to CDK4/6 inhibition in TNBC. CDK4/6 inhibitors, alone and in combination, are a novel therapeutic strategy for specific subgroups of TNBC. Clin Cancer Res; 23(18); 5561-72. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Nicholas C. Turner has received research funding from Pfizer and Roche and is a consultant/advisory board member for Novartis, Pfizer, and Roche. Deepak Sampath, Jennifer Arca Lacap, Lisa Crocker and Amy Young are employees of Genentech, Roche group. The other authors disclosed no potential conflicts of interest.
Figures




References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. - PubMed
-
- Finn RS, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. - PubMed
-
- Turner NC, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373(3):209–19. - PubMed
-
- Finn RS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375(20):1925–1936. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

